正在加载图片...
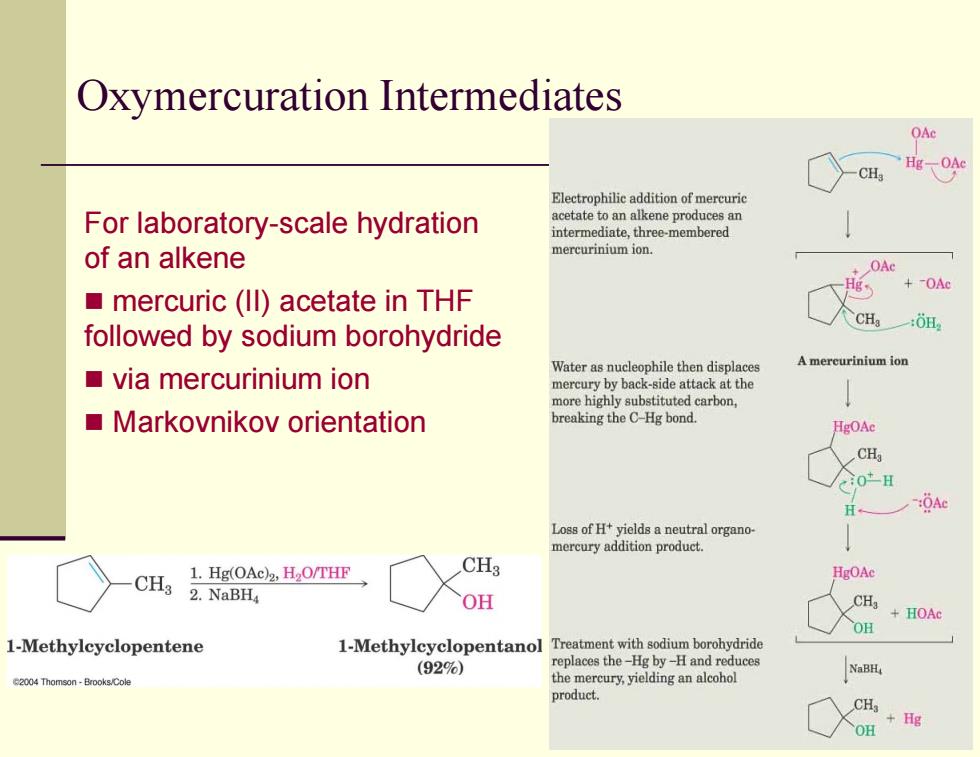
Oxymercuration Intermediates OAc CH Electrophilie addition of mercurie For laboratory-scale hydration acetate to an alkene produces an intermediate,three-membered of an alkene mercurinium ion. OAc +OAc mercuric(ll)acetate in THF CH followed by sodium borohydride Water as nucleophile then displaces A mercurinium ion ■via mercurinium ion mercury by back-side attack at the more highly substituted carbon, Markovnikov orientation breaking the C-Hg bond. HgOAc 0-H H OAe Loss of Ht yields a neutral organo- mercury addition product. 1.Hg(OAc)2,H2O/THF CH3 HgOAe CH3 2.NaBH OH CH +HOAc OH 1-Methylcyclopentene 1-Methylcyclopentanol Treatment with sodium borohydride (92%) replaces the-Hg by-H and reduces 004Thomson-Brooks.Cole themercuryying product. CH OH +HgOxymercuration Intermediates For laboratory-scale hydration of an alkene mercuric (II) acetate in THF followed by sodium borohydride via mercurinium ion Markovnikov orientation