正在加载图片...
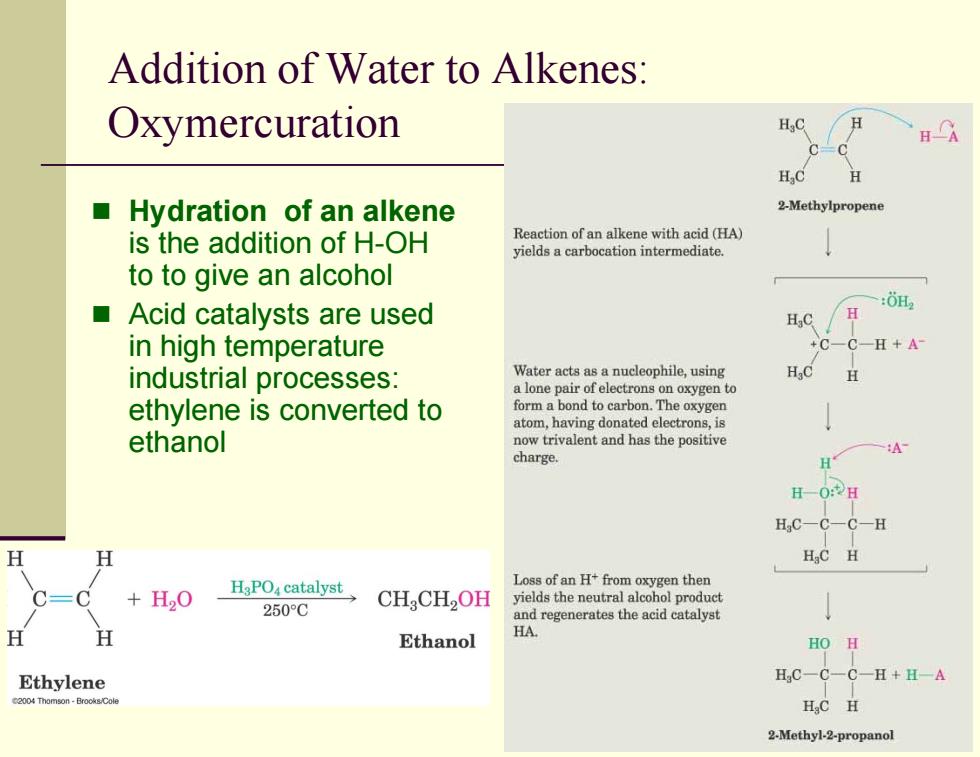
Addition of Water to Alkenes: Oxymercuration C=C H,C H Hydration of an alkene 2-Methylpropene is the addition of H-OH Reaction of an alkene with acid (HA) yields a carbocation intermediate. toto give an alcohol Acid catalysts are used :ǒH in high temperature +C一CH+A industrial processes: Water acts as a nucleophile,using H a lone pair of electrons on oxygen to ethylene is converted to form a bond to carbon.The oxygen atom,having donated electrons,is ethanol now trivalent and has the positive :A charge. H-0:2H HC-C一C-H H H HC H Loss of an H+from oxygen then +H20 H3PO,catalyst CHCH2OH yields the neutral alcohol product 250°C and regenerates the acid catalyst Ethanol HA. HO H Ethylene H:C-C-C-H+HA 24 Thomson-BrooksCole HC H 2-Methyl-2-propanol Addition of Water to Alkenes: Oxymercuration Hydration of an alkene is the addition of H-OH to to give an alcohol Acid catalysts are used in high temperature industrial processes: ethylene is converted to ethanol