正在加载图片...
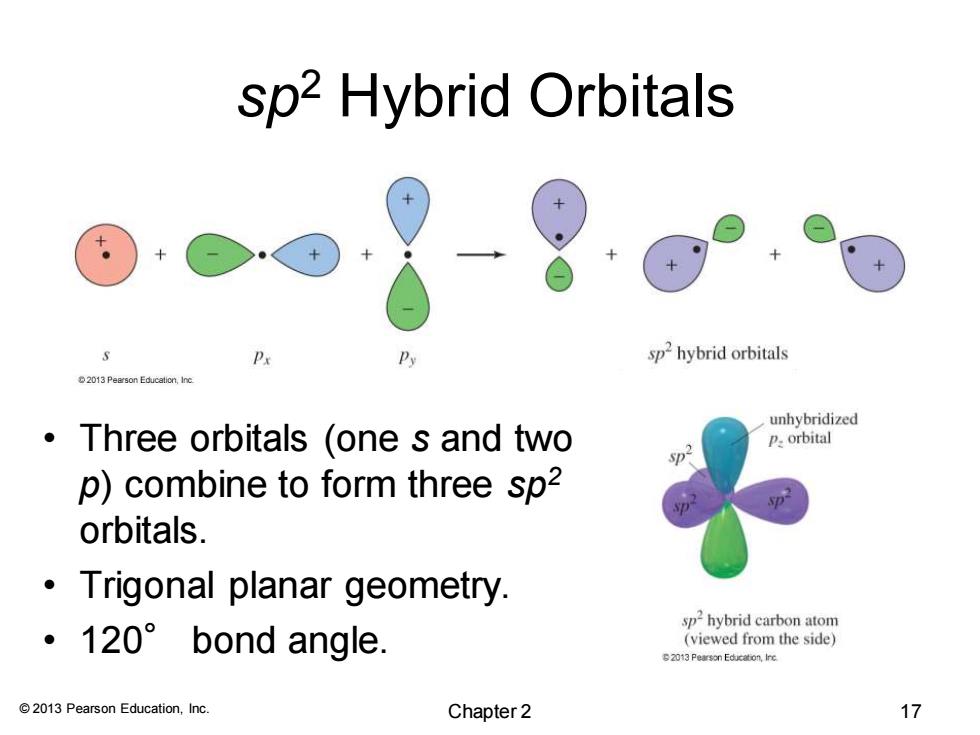
sp2 Hybrid Orbitals Px P sphybrid orbitals unhybridized Three orbitals (one s and two P.orbital p p)combine to form three sp2 orbitals. Trigonal planar geometry. sp2 hybrid carbon atom ·120°bond angle. (viewed from the side) 2013 Pearson Education,Inc. Chapter 2 17© 2013 Pearson Education, Inc. sp2 Hybrid Orbitals • Three orbitals (one s and two p) combine to form three sp2 orbitals. • Trigonal planar geometry. • 120° bond angle. Chapter 2 17