正在加载图片...
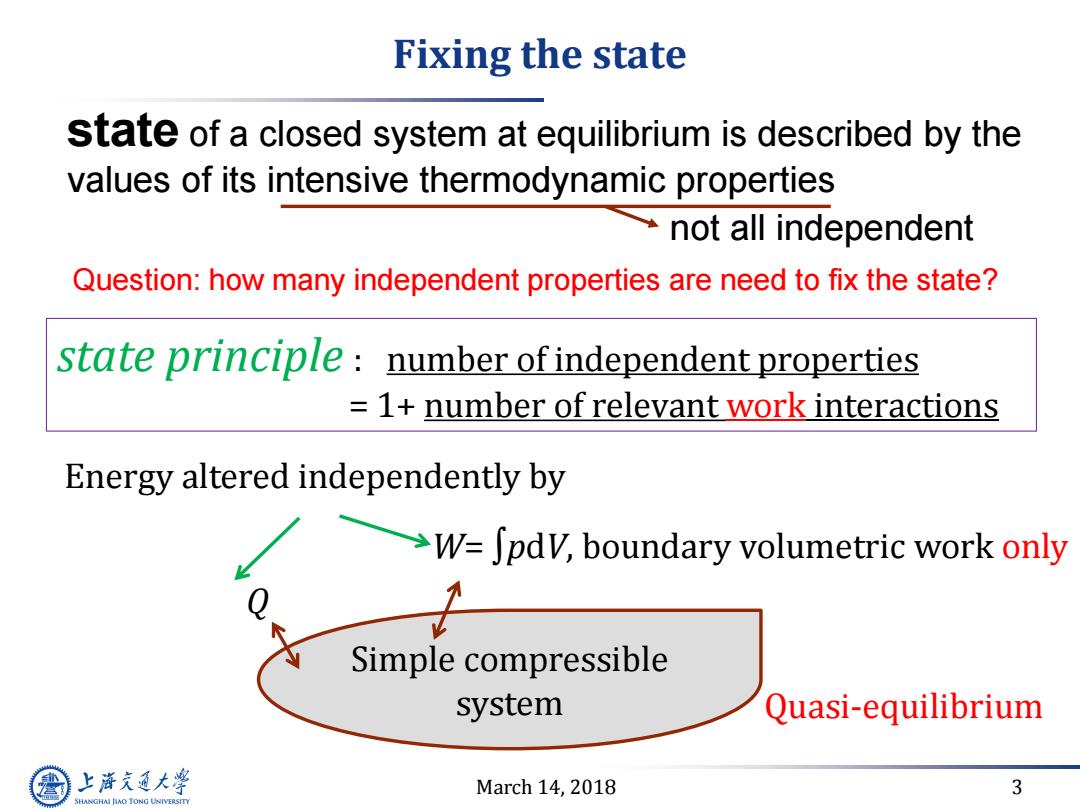
Fixing the state state of a closed system at equilibrium is described by the values of its intensive thermodynamic properties not all independent Question:how many independent properties are need to fix the state? state principle number of independent properties =1+number of relevant work interactions Energy altered independently by >W=Jpdv,boundary volumetric work only Simple compressible system Quasi-equilibrium 上游充通大 March 14,2018 3 SHANGHAI JIAO TONG UNIVERSITYMarch 14, 2018 3 Fixing the state Simple compressible system Quasi-equilibrium W= ∫pdV, boundary volumetric work only Q Energy altered independently by state principle : number of independent properties = 1+ number of relevant work interactions state of a closed system at equilibrium is described by the values of its intensive thermodynamic properties not all independent Question: how many independent properties are need to fix the state?