
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 9-10 Spring,3/14/2018 Prof.,Dr.Yonghua HUANG 强 Ann是n http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lecture 9-10 Spring, 3/14/2018 Prof., Dr. Yonghua HUANG http://cc.sjtu.edu.cn/G2S/site/thermo.html
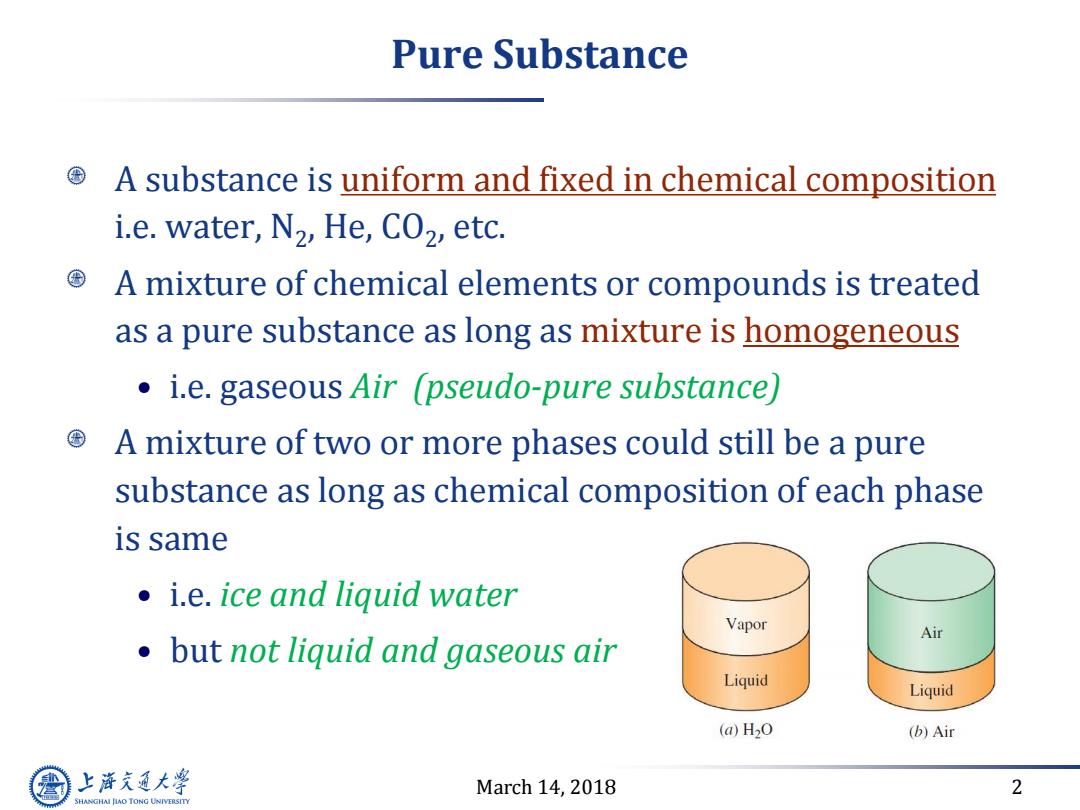
Pure Substance A substance is uniform and fixed in chemical composition i.e.water,N2,He,CO2,etc. A mixture of chemical elements or compounds is treated as a pure substance as long as mixture is homogeneous i.e.gaseous Air (pseudo-pure substance) A mixture of two or more phases could still be a pure substance as long as chemical composition of each phase is same i.e.ice and liquid water Vapor Air but not liquid and gaseous air Liquid Liquid (a)H20 (b)Air 上游通大学 March 14,2018 2 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 2 Pure Substance A substance is uniform and fixed in chemical composition i.e. water, N2 , He, CO2 , etc. A mixture of chemical elements or compounds is treated as a pure substance as long as mixture is homogeneous • i.e. gaseous Air (pseudo-pure substance) A mixture of two or more phases could still be a pure substance as long as chemical composition of each phase is same • i.e. ice and liquid water • but not liquid and gaseous air
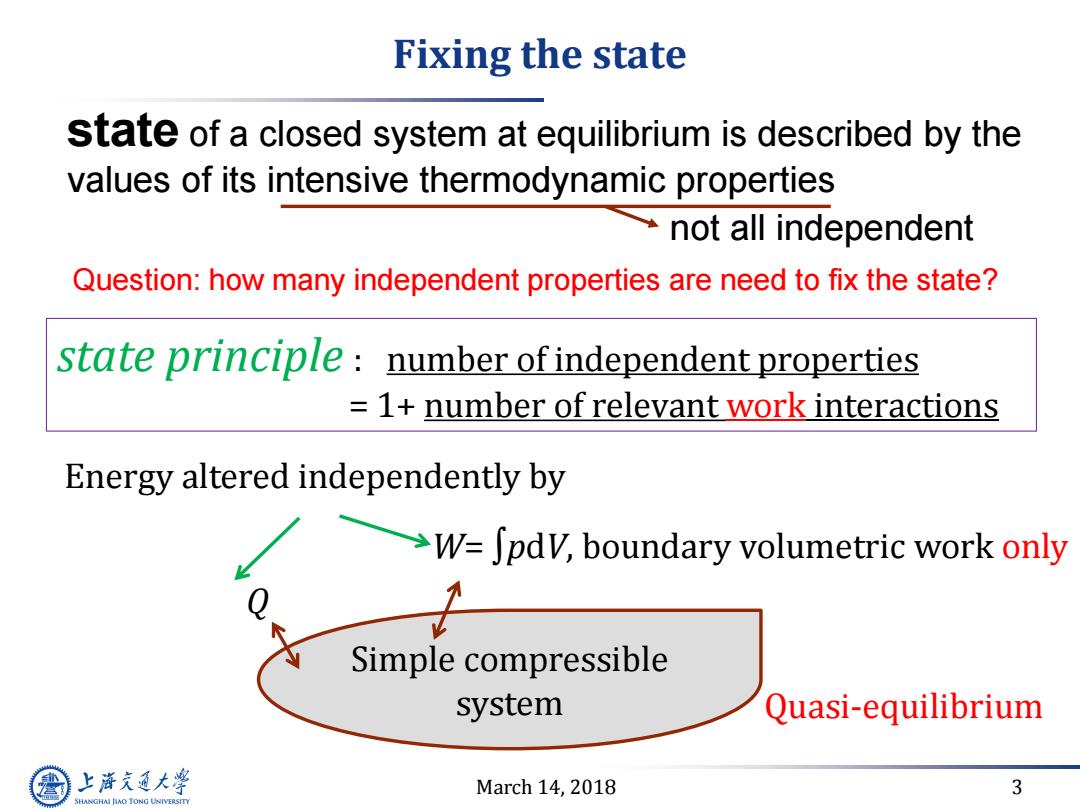
Fixing the state state of a closed system at equilibrium is described by the values of its intensive thermodynamic properties not all independent Question:how many independent properties are need to fix the state? state principle number of independent properties =1+number of relevant work interactions Energy altered independently by >W=Jpdv,boundary volumetric work only Simple compressible system Quasi-equilibrium 上游充通大 March 14,2018 3 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2018 3 Fixing the state Simple compressible system Quasi-equilibrium W= ∫pdV, boundary volumetric work only Q Energy altered independently by state principle : number of independent properties = 1+ number of relevant work interactions state of a closed system at equilibrium is described by the values of its intensive thermodynamic properties not all independent Question: how many independent properties are need to fix the state?
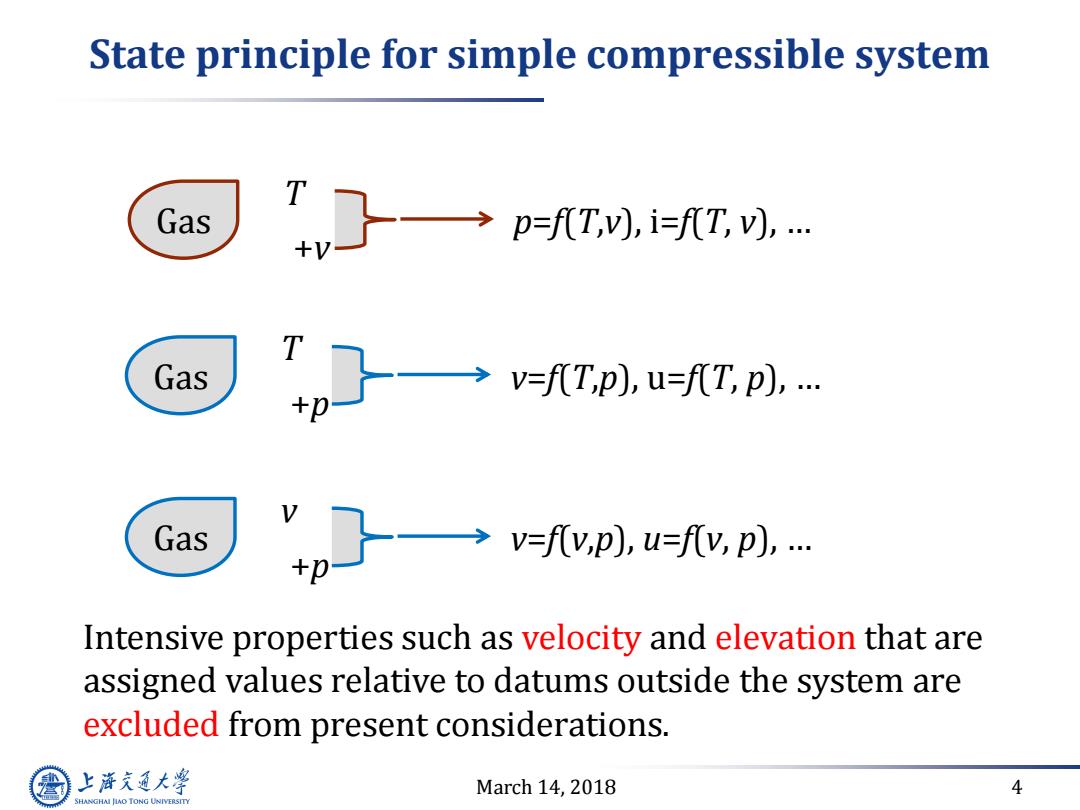
State principle for simple compressible system Gas →p=Ti7,W.… +V- Gas →fTp),u=fT,p),… Gas →V=fy,p),u=fv,p),… Intensive properties such as velocity and elevation that are assigned values relative to datums outside the system are excluded from present considerations. 上游充通大 March 14,2018 4 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 4 State principle for simple compressible system Gas T +v p=f(T,v), i=f(T, v), … Gas T +p v=f(T,p), u=f(T, p), … Gas v +p v=f(v,p), u=f(v, p), … Intensive properties such as velocity and elevation that are assigned values relative to datums outside the system are excluded from present considerations
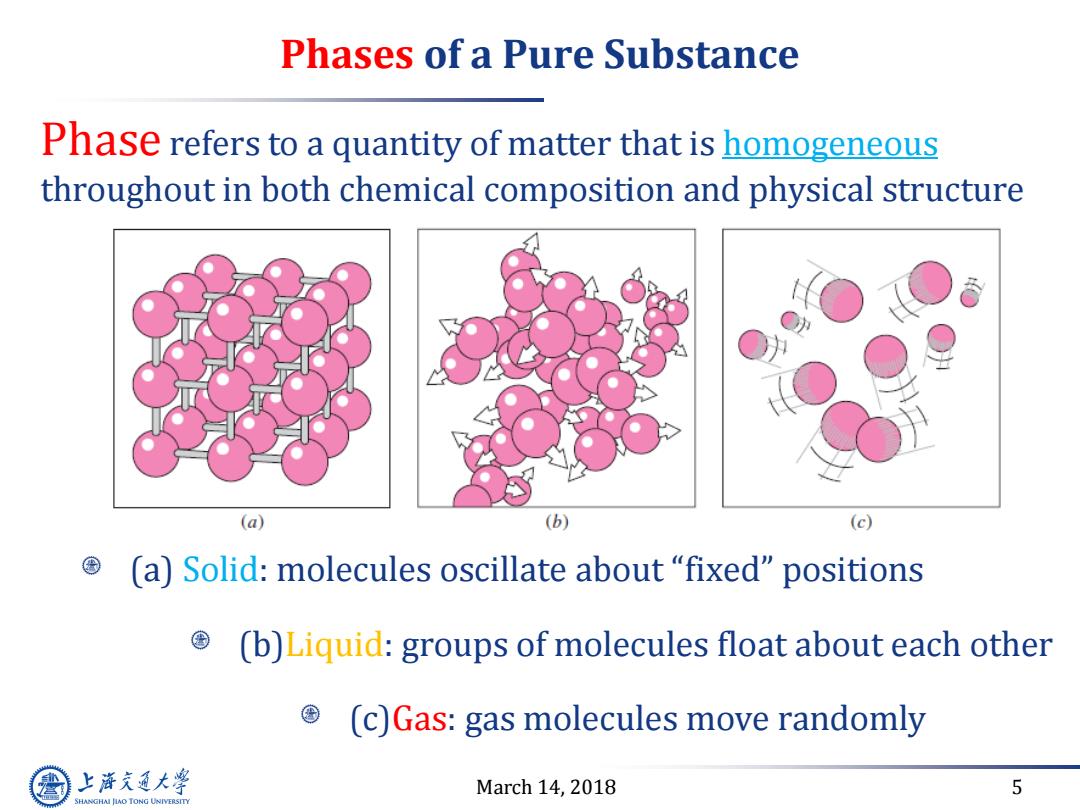
Phases of a Pure Substance Phase refers to a quantity of matter that is homogeneous throughout in both chemical composition and physical structure (a) (b) (c) (a)Solid:molecules oscillate about "fixed"positions (b)Liquid:groups of molecules float about each other (c)Gas:gas molecules move randomly 上游究通大学 March 14,2018 5 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 5 Phases of a Pure Substance (a) Solid: molecules oscillate about “fixed” positions (b)Liquid: groups of molecules float about each other (c)Gas: gas molecules move randomly Phase refers to a quantity of matter that is homogeneous throughout in both chemical composition and physical structure
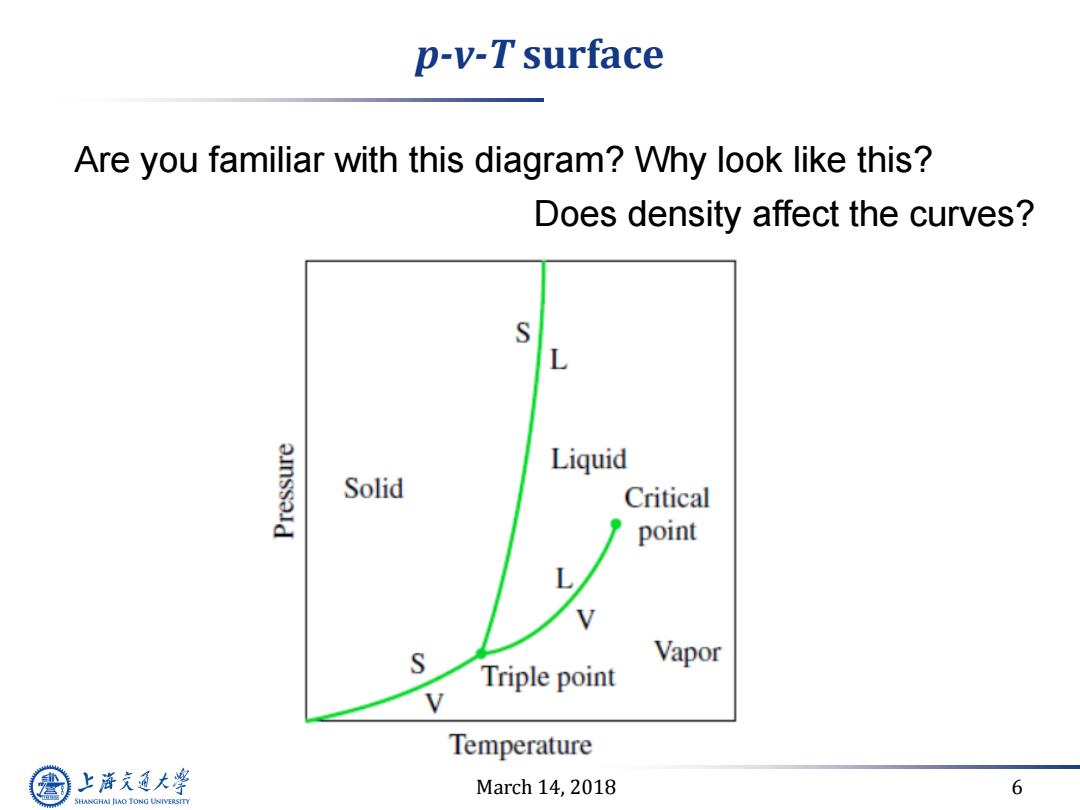
p-v-T surface Are you familiar with this diagram?Why look like this? Does density affect the curves? S L Liquid Solid Critical point L S Vapor Triple point Temperature 上游充通大 March 14,2018 6 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 6 p-v-T surface Are you familiar with this diagram? Why look like this? Does density affect the curves?
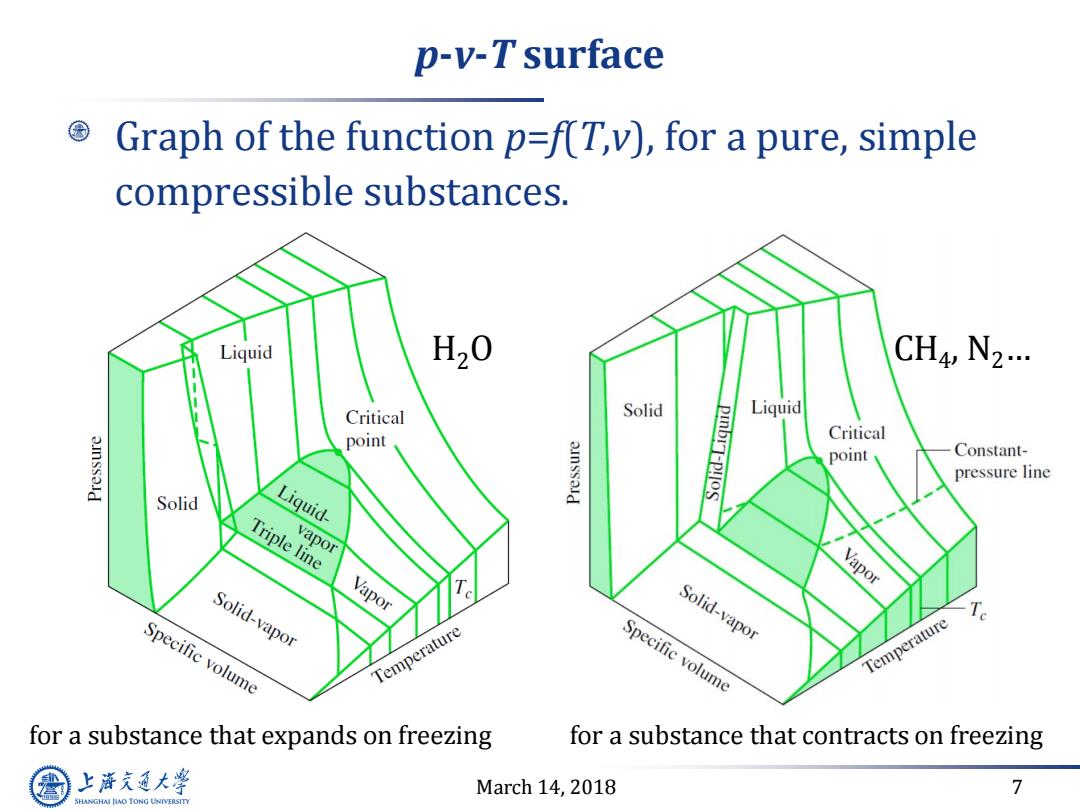
p-v-T surface Graph of the function p=f(T,v),for a pure,simple compressible substances. Liquid H20 CH4,N2… Critical Solid Liquid point pIbrT-P!oS Critical point Constant- pressure line Solid Liquid- Triple line vapor Solid-vapor Vapor Vapor Solid-vapor Specific volume Temperature Specific volume Temperature for a substance that expands on freezing for a substance that contracts on freezing 上降文通大学 March 14,2018 7 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 7 p-v-T surface Graph of the function p=f(T,v), for a pure, simple compressible substances. for a substance that expands on freezing for a substance that contracts on freezing H2O CH4 , N2…

Sculpture of p-v-T surface Maxwell's 3D Sketch of the lines on Gibbs' thermodynamic surface(8 July 1875) Gibbs'equation of state de=tdn-p du t,absolute temperature p.pressure Scottish engineer James Thomson's 1871 Gibbs'dagram sculptured by thermodynamics surface model,on display at James Clerk Maxwel 1874 the Hunterian Museum and Art Gallery,University of Glasgow Maxwell's thermodynamic surface,at Cambridge University,with 2007 overlay annotation added on by American engineer Ronald Kriz 上游充通大 March 14,2018 8 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 8 Sculpture of p-v-T surface Maxwell's 3D Sketch of the lines on Gibbs’ thermodynamic surface (8 July 1875) Scottish engineer James Thomson's 1871 thermodynamics surface model, on display at the Hunterian Museum and Art Gallery, University of Glasgow Maxwell's thermodynamic surface, at Cambridge University, with 2007 overlay annotation added on by American engineer Ronald Kriz

Reading the surface Single-phase regions:Solid,Liquid,Vapor. State fixed by 2 properties L-V dome Two-phase regions:. Liquid H20 Melting,vaporization,sublimation Critical Equilibrium:S-L,L-V,S-V point T,P not independent!!! Solid/ Liquid- vapbr · State fixed by v and (T or p) Solid Triple line Vapor ● Saturation states/lines Subcooled Solid-vapor (Compressed) Specific volume Temperature Triple line:S-L-V liquid H20: Saturated T,=273.16K liquid P=0.6113kPa Saturated Superheated Critical point:To pe ve Vapor /Gas vapor 上游充通大学 March 14,2018 9 SHANGHAI JLAO TONG UNIVERSITY
March 14, 2018 9 Reading the surface Single-phase regions: Solid, Liquid, Vapor. • State fixed by 2 properties Two-phase regions:. • Melting, vaporization, sublimation • Equilibrium: S-L, L-V, S-V • T, P not independent!!! • State fixed by v and (T or p) • Saturation states/lines Triple line: S-L-V Critical point: Tc , pc , vc H2O H2O : Tr =273.16K pr = 0.6113kPa L-V dome Saturated liquid Saturated vapor Subcooled (Compressed) liquid Superheated Vapor /Gas Solid
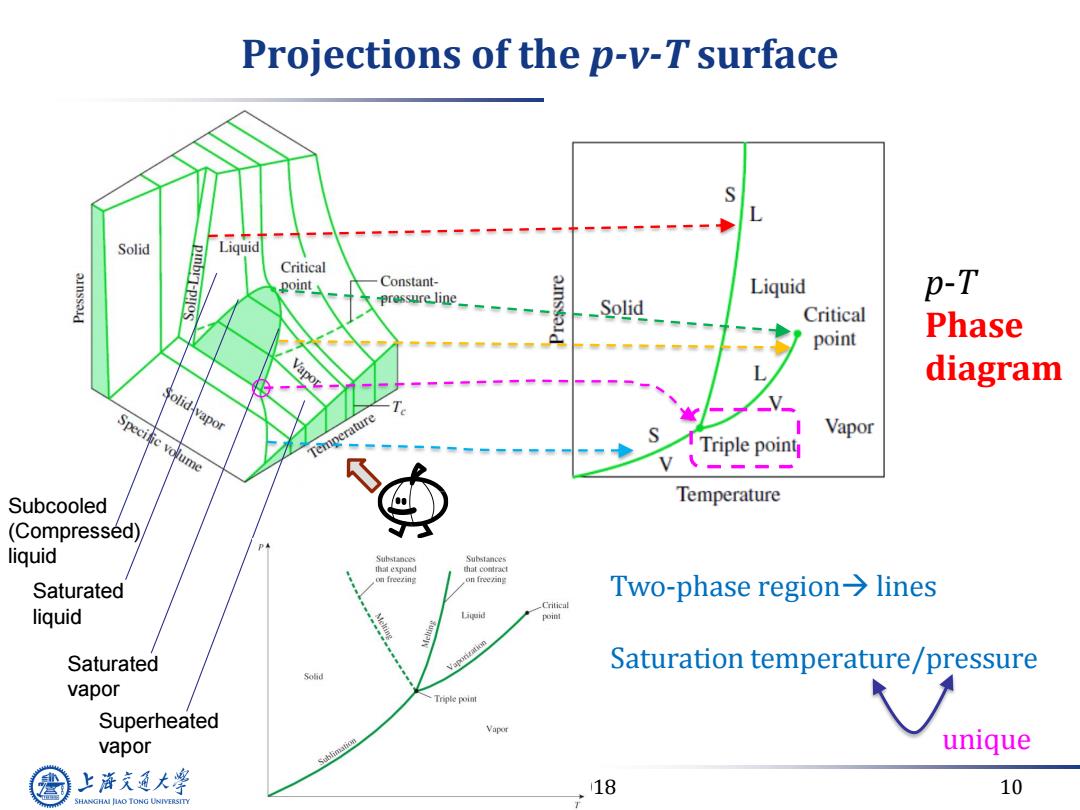
Projections of the p-v-T surface Solid Liquid -1 pInbr-Py Critical point Constant- Liquid pressure line p-T _Solid Critical point Phase diagram Solid vapor Specitic volume Temperature Triple point Vapor Subcooled Temperature (Compressed) liquid Substances Substances that expand rhat conract Saturated Two-phase region>lines Critical liquid Liyuid Saturated Saturation temperature/pressure Solid vapor Triple point Superheated Vapor vapor Sublimation unique 上降文通大学 18 10 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2018 10 Projections of the p-v-T surface p-T Phase diagram • Two-phase region lines • Saturation temperature/pressure unique Saturated liquid Saturated vapor Subcooled (Compressed) liquid Superheated vapor