
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 46 Chapter 9 Gas Power Cycles Spring,2017 Prof.,Dr.Yonghua HUANG 强 AR是r 目e http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lecture 46 Spring, 2017 Prof., Dr. Yonghua HUANG Chapter 9 Gas Power Cycles http://cc.sjtu.edu.cn/G2S/site/thermo.html
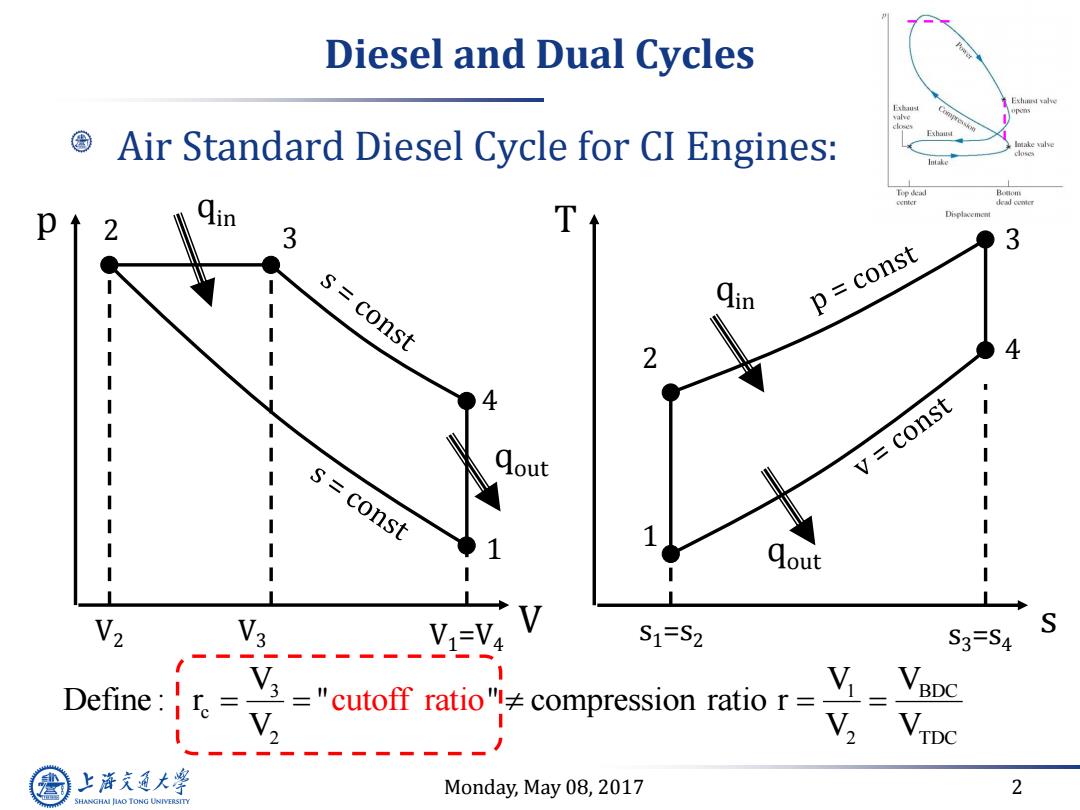
Diesel and Dual Cycles ns Exbaud Air Standard Diesel Cycle for CI Engines: Top dead p 2 3 s=const p const 4 const V s=const I 1 1 1 Qout V3 V1=V4 S1=S2 S3=S4 Define: Y3 ="cutoff ratio"compression ratio r= V, 八---22---2- 上降文通大学 Monday,May 08,2017 2 SHANGHAI JIAO TONG UNIVERSITY
Monday, May 08, 2017 2 Diesel and Dual Cycles Air Standard Diesel Cycle for CI Engines: qout s T 1 2 4 3 s1=s2 s3=s4 qin qout V p 1 2 4 3 V1=V4 qin V2 V3 3 BDC 1 c 2 2 TDC V V V Define : r " " compression ratio r V V cutoff ratio V
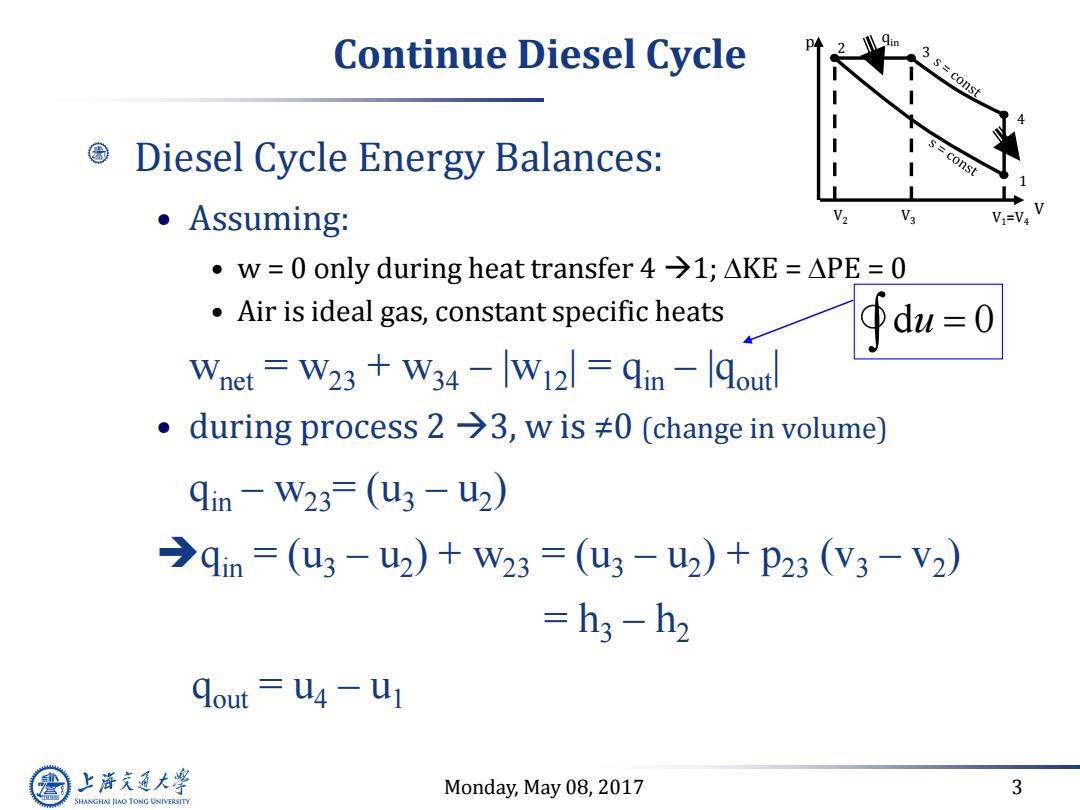
Continue Diesel Cycle s=const Diesel Cycle Energy Balances: const Assuming: V V3 V1=V4 ·w=0 only during heat transfer4→1;△KE=△PE=0 Air is ideal gas,constant specific heats du=0 Wnet W23 W34-W121 qin -lqoutl ·during process2→3,wis≠0(change in volume) qin-W23=(u3-u2) →qin=(u3-u2)+W23=(u3-u2)+P23(V3-V2) =h3-h2 dout U4 -U1 上游通大学 Monday,May 08,2017 3 SHANGHAI JIAO TONG UNIVERSITY
Monday, May 08, 2017 3 Continue Diesel Cycle Diesel Cycle Energy Balances: • Assuming: • w = 0 only during heat transfer 4 1; DKE = DPE = 0 • Air is ideal gas, constant specific heats wnet = w23 + w34 – |w12| = qin – |qout| • during process 2 3, w is ≠0 (change in volume) qin – w23= (u3 – u2 ) qin = (u3 – u2 ) + w23 = (u3 – u2 ) + p23 (v3 – v2 ) = h3 – h2 qout = u4 – u1 d 0 u V p 1 2 4 3 V1=V4 qin V2 V3
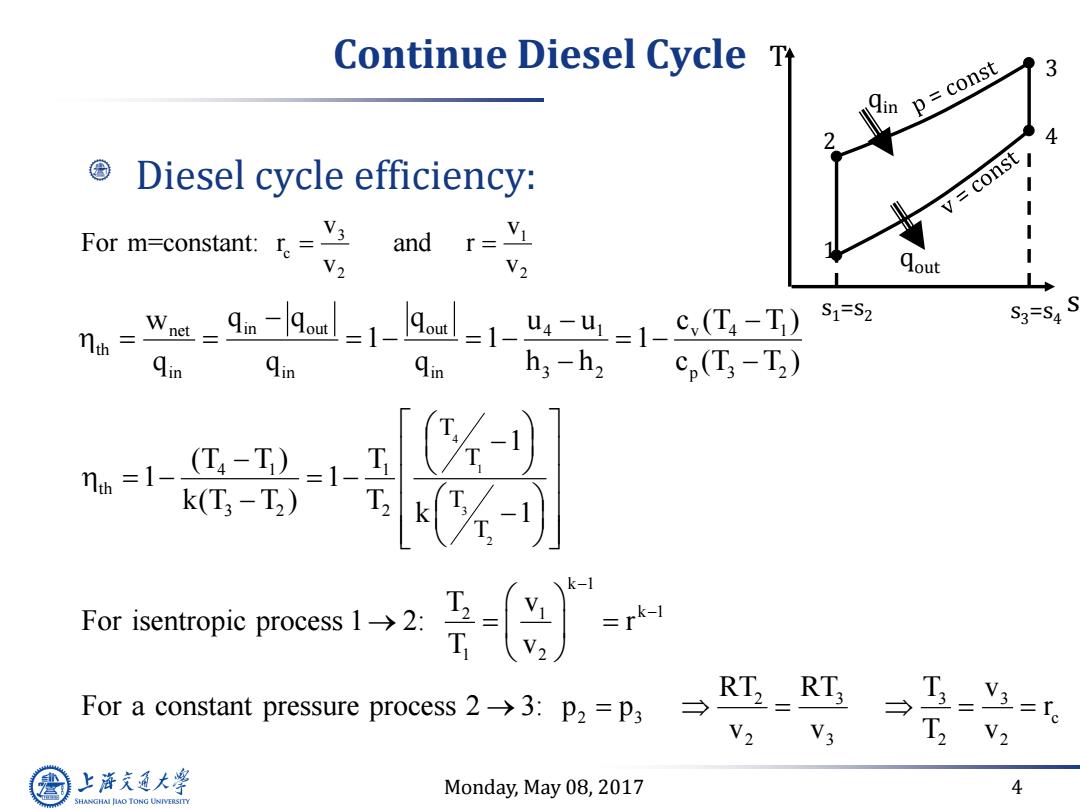
Continue Diesel Cycle T p=const Diesel cycle efficiency: v=const For m-constant:=v and r=V I V2 V2 Qout wu-9。-l9m-1-Aa-1-u-u=1-c,L-T )S1=S2 S3= Qin h3-h2 cp(T;-T2) k(T3-T2) k-1 For isentropie proces: =r For a constant pressure process2→3:p2=p3→ RT2 RT; T V2 V3 上游充通大 Monday,May 08,2017 4 SHANGHAI JIAO TONG UNIVERSITY
Monday, May 08, 2017 4 Continue Diesel Cycle Diesel cycle efficiency: 3 1 c 2 2 v v For m=constant: r and r v v s T 1 2 4 3 s1=s2 s3=s4 qin qout net v 4 1 in out out 4 1 th in in in 3 2 p 3 2 w c (T T ) q q q u u 1 1 1 q q q h h c (T T ) 4 1 3 2 4 1 1 th 3 2 2 T T T T 1 (T T ) T 1 1 k(T T ) T k 1 k 1 2 1 k 1 1 2 T v For isentropic process 1 2: r T v 2 3 3 3 2 3 c 2 3 2 2 RT RT T v For a constant pressure process 2 3: p p r v v T v
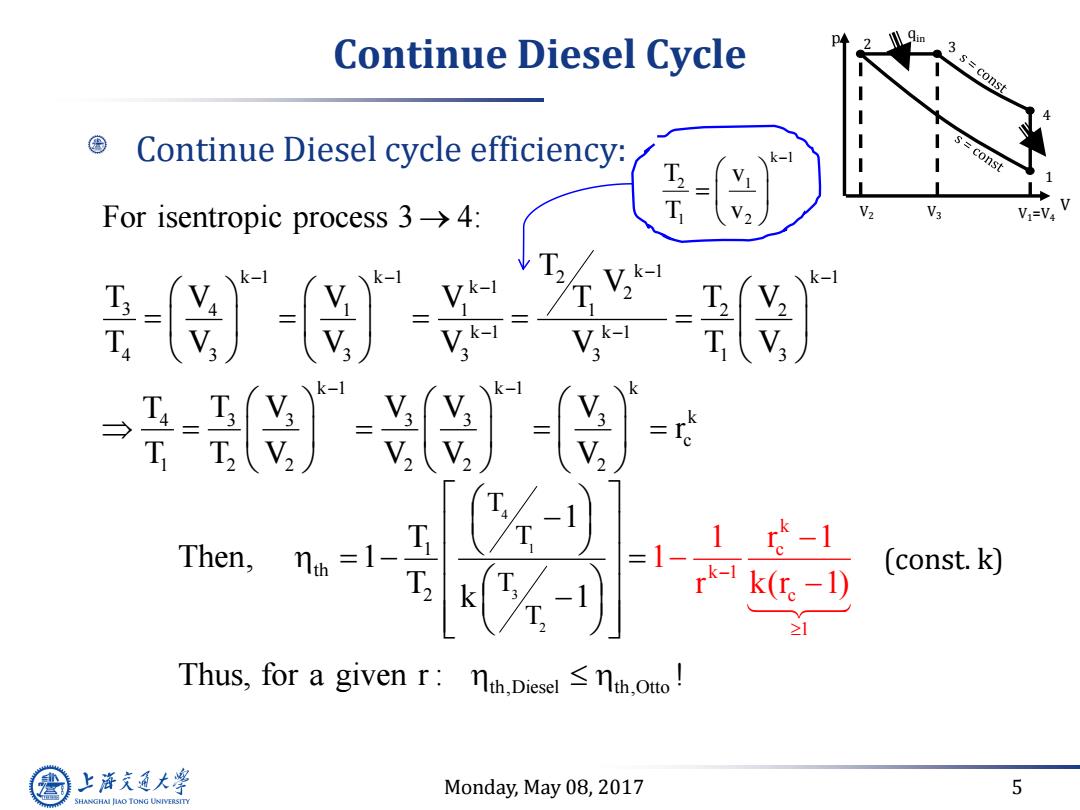
Continue Diesel Cycle 3 s const Continue Diesel cycle efficiency: const T For isentropic process3→4: T V2 V3 V1=V4 Then,r 1 =1- 1-1 T k。-1) (const.k) ≥1 Thus,for a given r:nt.Diesel <nth.o! 上游通大学 Monday,May 08,2017 5 SHANGHAI JLAO TONG UNIVERSITY
Monday, May 08, 2017 5 Continue Diesel Cycle Continue Diesel cycle efficiency: k 1 k 1 k 1 k 1 2 k 1 2 3 4 1 1 1 2 2 k 1 k 1 4 3 3 3 3 1 3 k 1 k 1 k 4 3 3 3 3 3 k c 1 2 2 2 2 2 For isentropic process 3 4: T V T V V V T T V T V V V V T V T T V V V V r T T V V V V k 1 2 1 1 2 T v T v (const. k) 4 1 3 2 k c k 1 th 2 1 c 1 T T T T 1 T Then, 1 T k 1 1 r 1 1 r k(r 1) V p 1 2 4 3 V1=V4 qin V2 V3 Thus, for a given r : ! th,Diesel th,Otto

Continue Diesel Cycle Continue Diesel cycle efficiency: T V=const_-一,3oto 3piesel p const 4 const for a given r:mth.Diesel Tth.o V I I I Qout S1=S2 S3=S4 However,Diesel cycle typically operates at higher r! 上降文通大学 Monday,May 08,2017 6 SHANGHAI JIAO TONG UNIVERSITY
Monday, May 08, 2017 6 Continue Diesel Cycle Continue Diesel cycle efficiency: s T 1 2 4 s1=s2 s3=s4 qin qout 3Diesel 3Otto th,Diesel th,Otto for a given r : ! However, Diesel cycle typically operates at higher r!
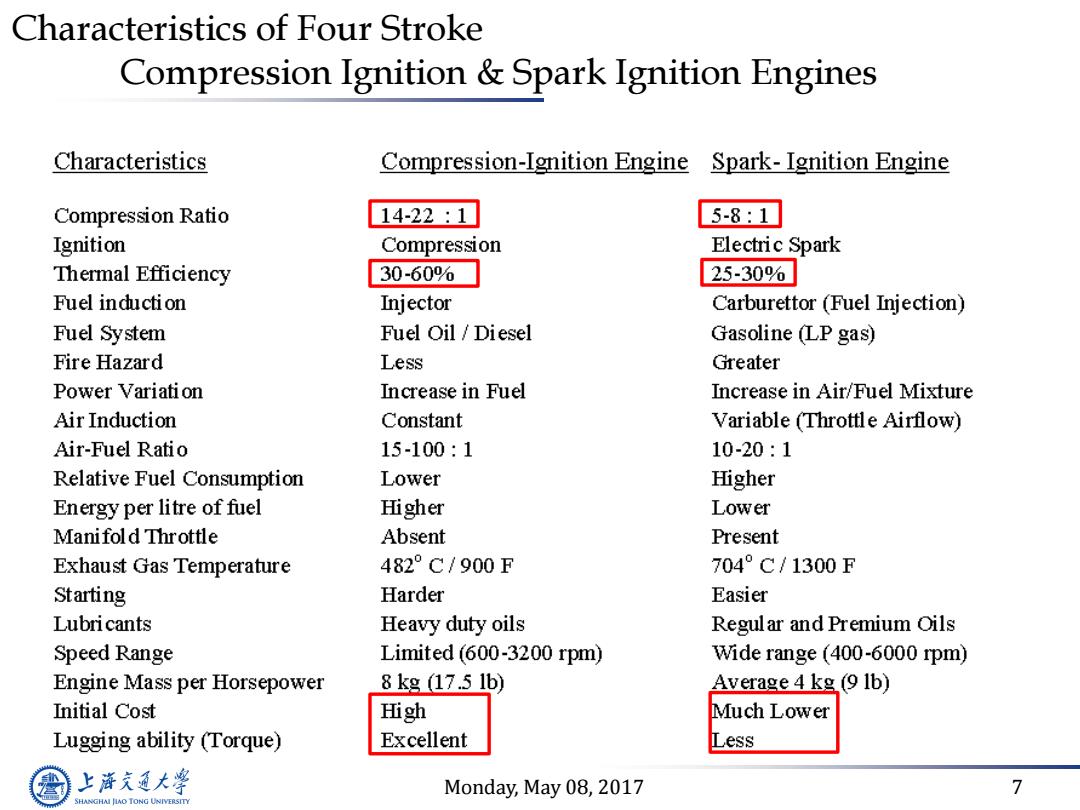
Characteristics of Four Stroke Compression Ignition Spark Ignition Engines Characteristics Compression-Ignition Engine Spark-Ignition Engine Compression Ratio 14-22:1 5-8:1 Ignition Compression Electric Spark Thermal Efficiency 30-60% 25-30% Fuel induction Injector Carburettor (Fuel Injection) Fuel System Fuel Oil /Diesel Gasoline LP gas) Fire Hazard Less Greater Power Variation Increase in Fuel Increase in Air/Fuel Mixture Air Induction Constant Variable(Throttle Airflow) Air-Fuel Ratio 15-100:1 10-20:1 Relative Fuel Consumption Lower Higher Energy per litre of fuel Higher Lower Manifold Throttle Absent Present Exhaust Gas Temperature 482°C/900F 704°C/1300F Starting Harder Easier Lubricants Heavy duty oils Regular and Premium Oils Speed Range Limited(600-3200 rpm) Wide range (400-6000 rpm) Engine Mass per Horsepower 8kg17.51b) Average 4 kg(9 1b) Initial Cost High Much Lower Lugging ability (Torque) Excellent Less 上游充通大学 Monday,May 08,2017 7 SHANGHAI JIAO TONG UNIVERSITY
Monday, May 08, 2017 7 Characteristics of Four Stroke Compression Ignition & Spark Ignition Engines
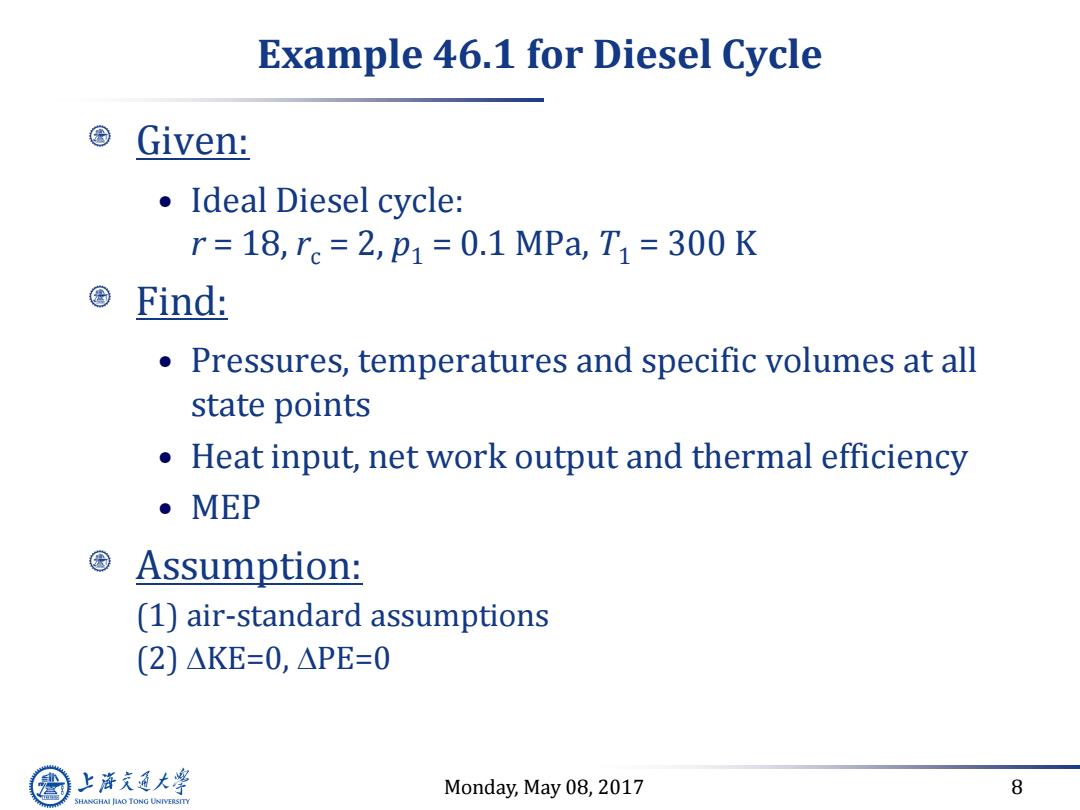
Example 46.1 for Diesel Cycle Given: ·Ideal Diesel cycle: r=18,re=2,p1=0.1MPa,T1=300K Find: Pressures,temperatures and specific volumes at all state points Heat input,net work output and thermal efficiency ●MEP Assumption: (1)air-standard assumptions (2)△KE=0,△PE=0 上游通大学 Monday,May 08,2017 8 SHANGHAI JLAO TONG UNIVERSITY
Monday, May 08, 2017 8 Example 46.1 for Diesel Cycle Given: • Ideal Diesel cycle: r = 18, rc = 2, p1 = 0.1 MPa, T1 = 300 K Find: • Pressures, temperatures and specific volumes at all state points • Heat input, net work output and thermal efficiency • MEP Assumption: (1) air-standard assumptions (2) DKE=0, DPE=0

Continue Example 46.1 Solution: D 2 3 T 3 V3=2 Ye-V2 02 s=c p=c S=C 4 U=C 巧 =18 P1 =0.1 MPa T1=300K U Assumptions: 1.The air in the piston-cylinder assembly is the closed system. 2.The compression and expansion processes are adiabatic. 3.All processes are internally reversible. 4.The air is modeled as an ideal gas. 5.Kinetic and potential energy effects are negligible. 上降文通大学 Monday,May 08,2017 9 SHANGHAI JLAO TONG UNIVERSITY
Monday, May 08, 2017 9 Continue Example 46.1 Solution: Assumptions: 1. The air in the piston–cylinder assembly is the closed system. 2. The compression and expansion processes are adiabatic. 3. All processes are internally reversible. 4. The air is modeled as an ideal gas. 5. Kinetic and potential energy effects are negligible
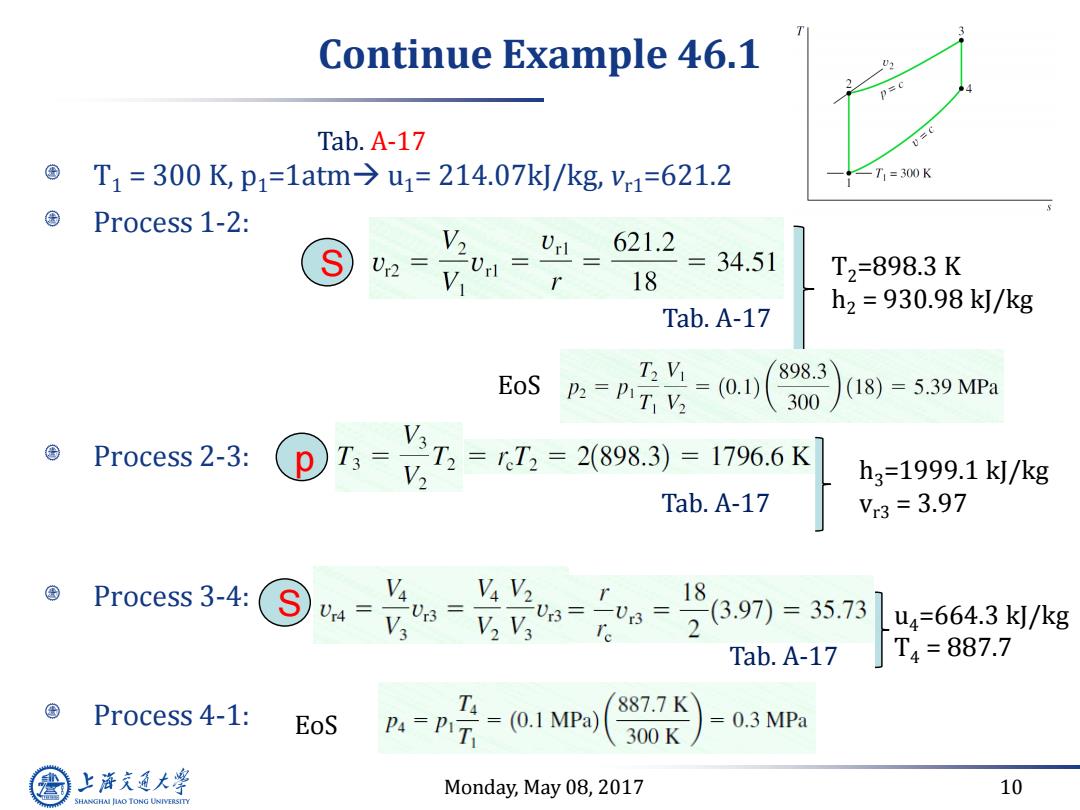
Continue Example 46.1 p=c Tab.A-17 v=c T1=300K,p1=1atm→u1=214.07k/kg,V1=621.2 ∠T1=300K Process 1-2: 621.2 =34.51 18 T2=898.3K Tab.A-17 h2=930.98k/kg EoS T2Vi P2=PIT V2 (0.0 898.3 (18)=5.39MPa 300 Process 2-3: T3= 7,=7,=28983)=1796.6K V h3=1999.1k/kg Tab.A-17 Vr3=3.97 Process 3-4: V Va V2 23.97)=35.73 1 U4= 3= V3 V2 V3 3=03= u4=664.3k/kg Tab.A-17 T4=887.7 Process 4-1: 887.7K EoS P4=PIT -0.1MPa) 0.3 MPa 300K 上游充通大 Monday,May 08,2017 10 SHANGHAI JLAO TONG UNIVERSITY
Monday, May 08, 2017 10 Continue Example 46.1 T1 = 300 K, p1=1atm u1= 214.07kJ/kg, vr1=621.2 Process 1-2: Process 2-3: Process 3-4: Process 4-1: Tab. A-17 S Tab. A-17 T2=898.3 K h2 = 930.98 kJ/kg EoS p Tab. A-17 h3=1999.1 kJ/kg vr3 = 3.97 S u4=664.3 kJ/kg T4 = 887.7 Tab. A-17 EoS