
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics (I) Spring,2018 Prof.,Dr.Yonghua HUANG 强 A Office Hour:Appointment(ME-A432) Email huangyh@sjtu.edu.cn;Office Phone:34206295
Engineering Thermodynamics (I) Spring, 2018 Prof., Dr. Yonghua HUANG Office Hour: Appointment(ME-A432) Email :huangyh@sjtu.edu.cn; Office Phone: 34206295

Modern vehicles 行日重加刻海前 然第■第性器 不H熊鼠 上游充通大学 February 28,2018 2 SHANGHAI JIAO TONG UNIVERSITY
February 28, 2018 2 Modern vehicles

Example:Renewable/green energy age Wind Solar tidal energy Nuclear fusion Nuclear fission power plant Hybrid diesel-electric locomotive,4400 HP 2010 15%increase in n 50%lower emissions 上游充通大学 February 28,2018 3 SHANGHAI JLAO TONG UNIVERSITY
February 28, 2018 3 Example: Renewable/green energy age Nuclear fusion Nuclear fission power plant Wind + Solar + tidal energy
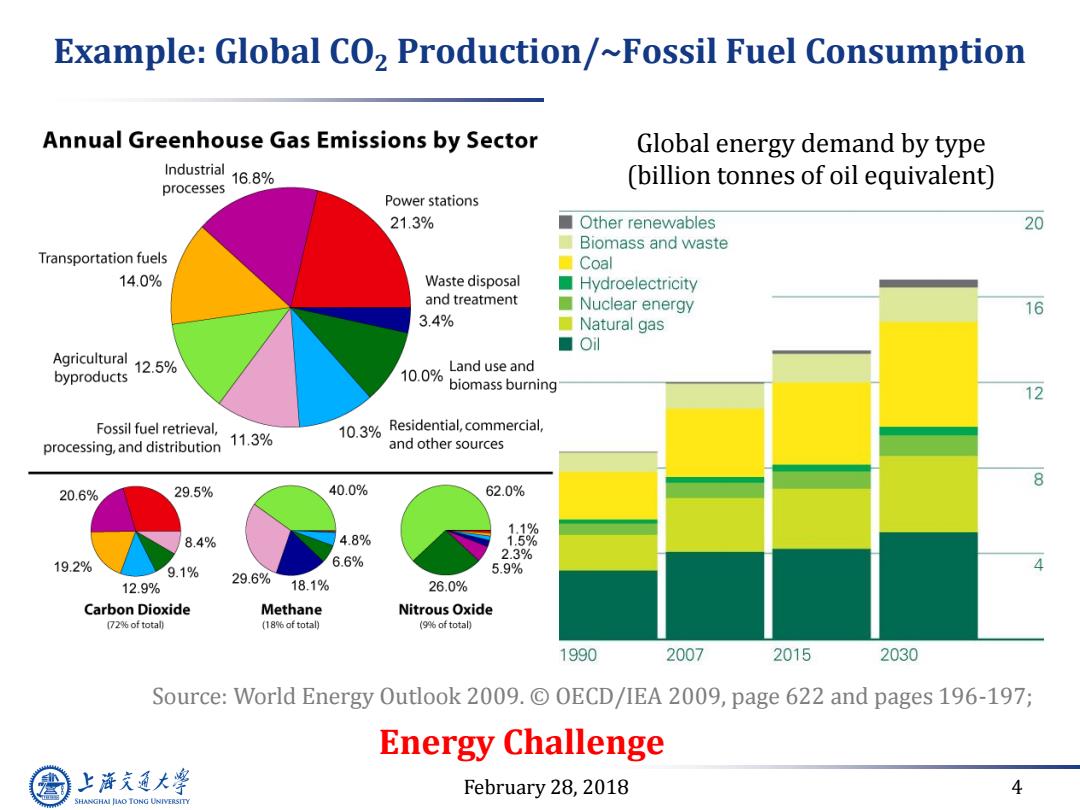
Example:Global CO2 Production/~Fossil Fuel Consumption Annual Greenhouse Gas Emissions by Sector Global energy demand by type Industrial 16.8% (billion tonnes of oil equivalent) processes Power stations 21.3% ■ Other renewables 20 Biomass and waste Transportation fuels 口Coal 14.0% Waste disposal ■ Hydroelectricity and treatment Nuclear energy 16 3.4% Natural gas ■Ol Agricultural byproducts 12.5% 10.0% Land use and biomass burning 12 Fossil fuel retrieval, ronanddisibtin1% 10.3%Residential,commercial, and other sources 8 20.6% 29.5% 40.0% 62.0% 8.4% 4.8% 19.2% 6.6% 2.3% 9.1% 5.9% 29.6% 12.9% 18.1% 26.0% Carbon Dioxide Methane Nitrous Oxide (72%of total) (18%of total) (9%of total) 1990 2007 2015 2030 Source:World Energy Outlook 2009.C OECD/IEA 2009,page 622 and pages 196-197; Energy Challenge 上降文通大学 February 28,2018 4 SHANGHAI JIAO TONG UNIVERSITY
February 28, 2018 4 Example: Global CO2 Production/~Fossil Fuel Consumption Global energy demand by type (billion tonnes of oil equivalent) Source: World Energy Outlook 2009. © OECD/IEA 2009, page 622 and pages 196-197; Energy Challenge
Two experiments What comes to your mind? Experiment #1 © Volume changing?Mass?Density?Pressure?Work? Energy? Liquid nitrogen?Why liquid?Temperature?T-V relation of gaseous air?Property?Cold?Warm?Heat Transfer? Volume changing?Work?Thermal EnergyWork? Experiment #2 Startup (transient)-steady?Volume changing?Work output?Electricity?Continuous running>cycle?Energy conversion?Combustion? Friction?Efficiency?Why high temperature? 上游充通大 February 28,2018 5 SHANGHAI JLAO TONG UNIVERSITY
February 28, 2018 5 Two experiments Volume changing? Mass? Density? Pressure? Work? Energy? Liquid nitrogen? Why liquid? Temperature? T-V relation of gaseous air? Property? Cold? Warm? Heat Transfer? Volume changing? Work? Thermal EnergyWork? What comes to your mind? Startup (transient) – steady? Volume changing? Work output? Electricity? Continuous runningcycle? Energy conversion? Combustion? Friction? Efficiency? Why high temperature? Experiment #2 Experiment #1

Other daily life problems? 40 km 4km Gas pipe line Gas Station x ol Gas cooker Gas cooker xo Your home My home 上游充通大 February 28,2018 6 SHANGHAI JIAO TONG UNIVERSITY
February 28, 2018 6 Other daily life problems?
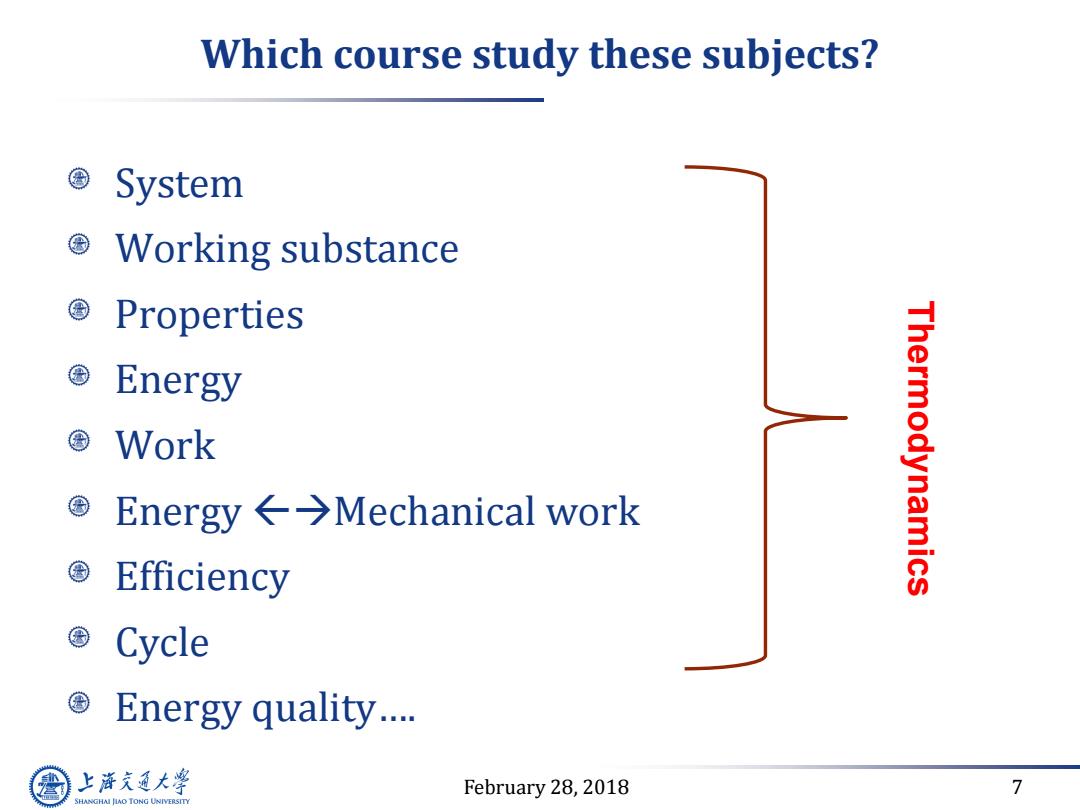
Which course study these subjects? System Working substance Properties Energy 国Work Energy←→Mechanical work Thermodynamics Efficiency Cycle Energy quality.… 上游充通大学 February 28,2018 7 SHANGHAI JLAO TONG UNIVERSITY
February 28, 2018 7 Which course study these subjects? System Working substance Properties Energy Work Energy Mechanical work Efficiency Cycle Energy quality…. Thermodynamics
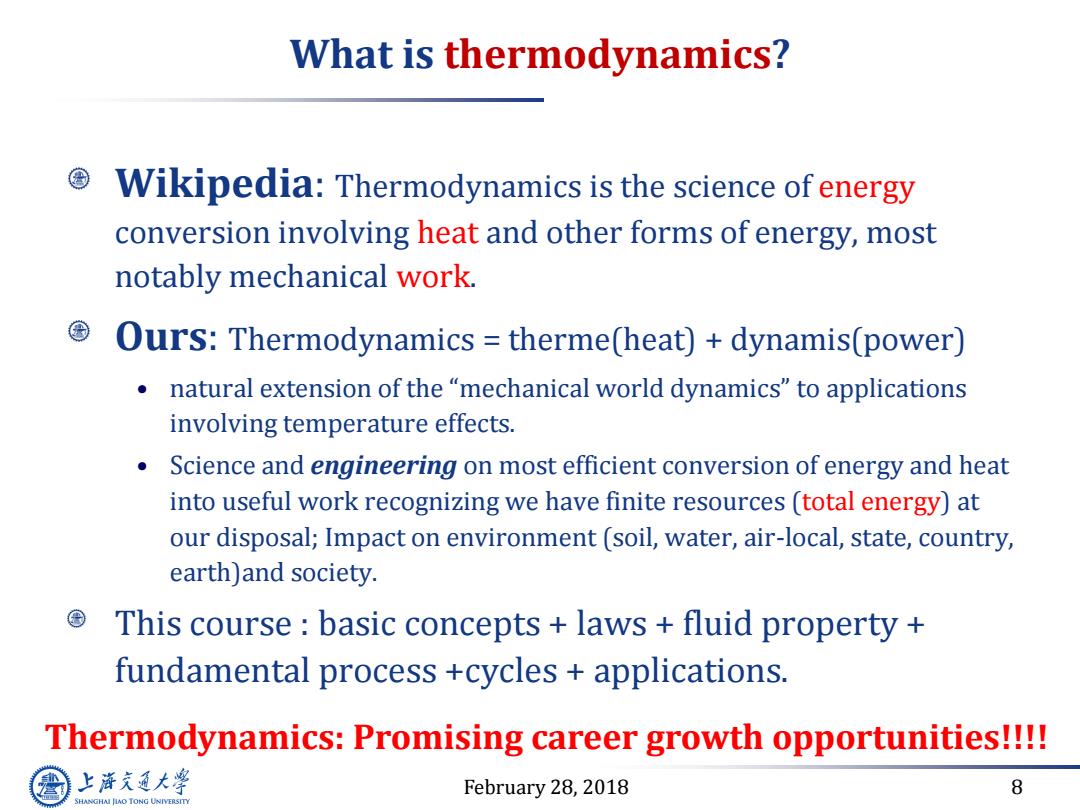
What is thermodynamics? Wikipedia:Thermodynamics is the science of energy conversion involving heat and other forms of energy,most notably mechanical work. Ours:Thermodynamics therme(heat)+dynamis(power) natural extension of the"mechanical world dynamics"to applications involving temperature effects. Science and engineering on most efficient conversion of energy and heat into useful work recognizing we have finite resources (total energy)at our disposal;Impact on environment (soil,water,air-local,state,country, earth)and society. This course basic concepts laws fluid property fundamental process +cycles applications. Thermodynamics:Promising career growth opportunities!!!! 上游充通大 February 28,2018 8 SHANGHAI JLAO TONG UNIVERSITY
February 28, 2018 8 What is thermodynamics? Wikipedia: Thermodynamics is the science of energy conversion involving heat and other forms of energy, most notably mechanical work. Ours: Thermodynamics = therme(heat) + dynamis(power) • natural extension of the “mechanical world dynamics” to applications involving temperature effects. • Science and engineering on most efficient conversion of energy and heat into useful work recognizing we have finite resources (total energy) at our disposal; Impact on environment (soil, water, air-local, state, country, earth)and society. This course : basic concepts + laws + fluid property + fundamental process +cycles + applications. Thermodynamics: Promising career growth opportunities!!!!
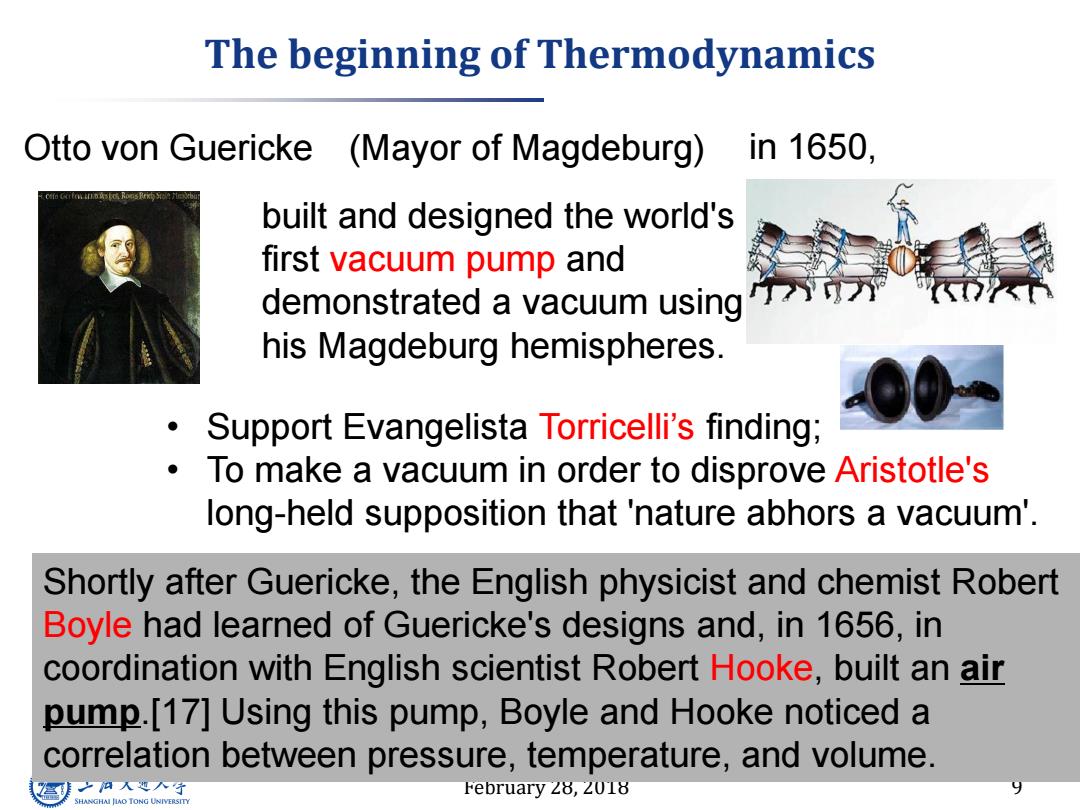
The beginning of Thermodynamics Otto von Guericke (Mayor of Magdeburg) in1650, built and designed the world's first vacuum pump and demonstrated a vacuum using his Magdeburg hemispheres. Support Evangelista Torricelli's finding; To make a vacuum in order to disprove Aristotle's long-held supposition that 'nature abhors a vacuum'. Shortly after Guericke,the English physicist and chemist Robert Boyle had learned of Guericke's designs and,in 1656,in coordination with English scientist Robert Hooke,built an air pump.[17]Using this pump,Boyle and Hooke noticed a correlation between pressure,temperature,and volume. 到之人入子 February 28,2018 9 SHANGHAI JLAO TONG UNIVERSITY
February 28, 2018 9 The beginning of Thermodynamics Otto von Guericke built and designed the world's first vacuum pump and demonstrated a vacuum using his Magdeburg hemispheres. • Support Evangelista Torricelli’s finding; • To make a vacuum in order to disprove Aristotle's long-held supposition that 'nature abhors a vacuum'. (Mayor of Magdeburg) in 1650, Shortly after Guericke, the English physicist and chemist Robert Boyle had learned of Guericke's designs and, in 1656, in coordination with English scientist Robert Hooke, built an air pump.[17] Using this pump, Boyle and Hooke noticed a correlation between pressure, temperature, and volume

The beginning of Thermodynamics Do you know these faces? James Watt,Scottish Sadi Carnot,French (6/11796-8/241832) James Joule,English (1/191736-8/251819) "Father of thermodynamics" (12/241818-10/111889) William Thomson,English Rudolf Clausius,German (6/271824-12/171907) Walther Nernst,German (2/21822-8/241888) 图上济文大学 1st 2nd law,Kelvin (6/251864-11/181941) 'entropy" February 28,2018 3rd law 10 SHANGHAI JIAO TONG UNIVERSITY
February 28, 2018 10 The beginning of Thermodynamics James Watt, Scottish (1/19 1736 –8/25 1819) James Joule, English (12/24 1818 –10/11 1889) Sadi Carnot, French (6/1 1796 –8/24 1832) “Father of thermodynamics" Rudolf Clausius, German (2/2 1822 –8/24 1888) “entropy” William Thomson, English (6/27 1824 –12/17 1907) 1 st & 2 nd law, Kelvin Walther Nernst, German (6/25 1864 –11/18 1941) 3 rd law Do you know these faces?