上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 23-24 Chapter 6 The second law of Thermodynamics (Section6.1-6.3,6.6) Spring,4/8/2018 强 Prof.,Dr.Yonghua HUANG A http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 23-24 Spring, 4/8/2018 Prof., Dr. Yonghua HUANG Chapter 6 The second law of Thermodynamics (Section 6.1- 6.3, 6.6) http://cc.sjtu.edu.cn/G2S/site/thermo.html
Why need the second law? First law-energy conservation (Quantity) dt How about the Quality of the energy? 中同人长第 4C61676666 C61676656 100 100 好好学习 上游究通大学 April 8,2018 2 SHANGHAI JIAO TONG UNIVERSITY
April 8, 2018 2 Why need the second law? First law – energy conservation How about the Quality of the energy? (Quantity)
Quality of energy 1 kJ electricity power <-1kJ heat 1 kJ chemical energy <-1kJ heat 300 上游充通大学 April 8,2018 3 SHANGHAI JLAO TONG UNIVERSITY
April 8, 2018 3 Quality of energy 1 kJ electricity power 1kJ heat 1 kJ chemical energy 1kJ heat
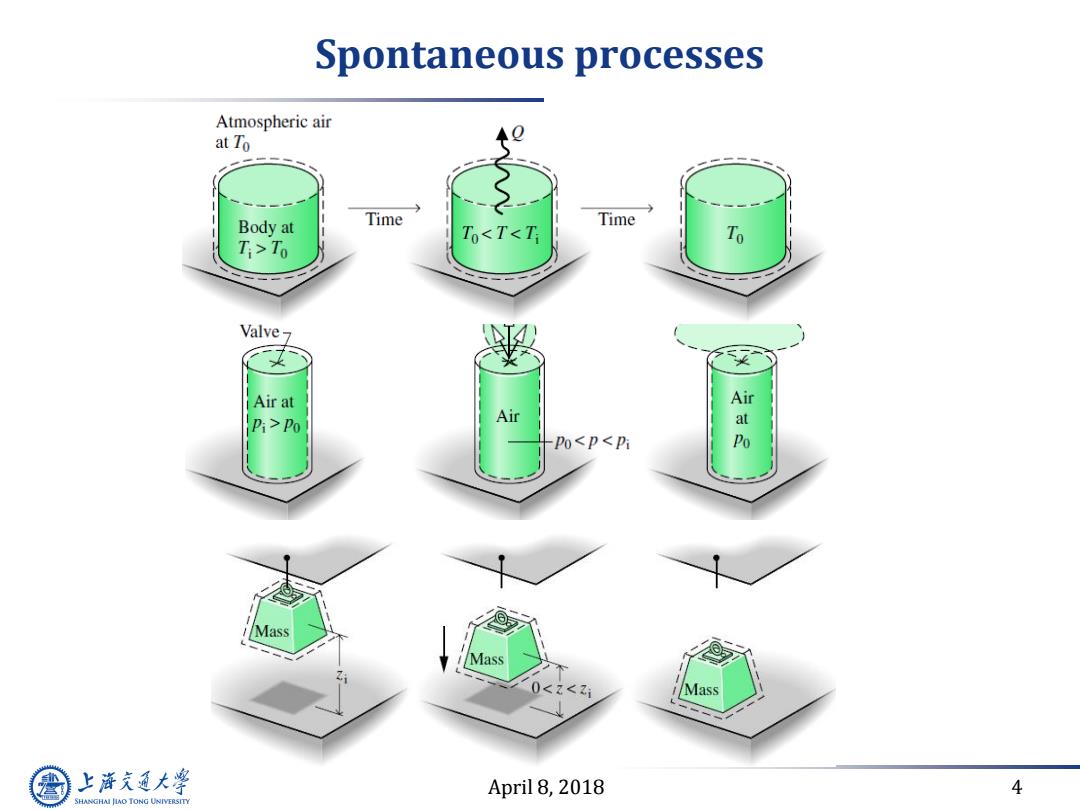
Spontaneous processes Atmospheric air at To Body at Time Time ToT0 Valve7 Air at Air Pi>Po Air at po<p<pi Po Mass Z< Mass 上游充通大学 April 8,2018 4 SHANGHAI JIAO TONG UNIVERSITY
April 8, 2018 4 Spontaneous processes
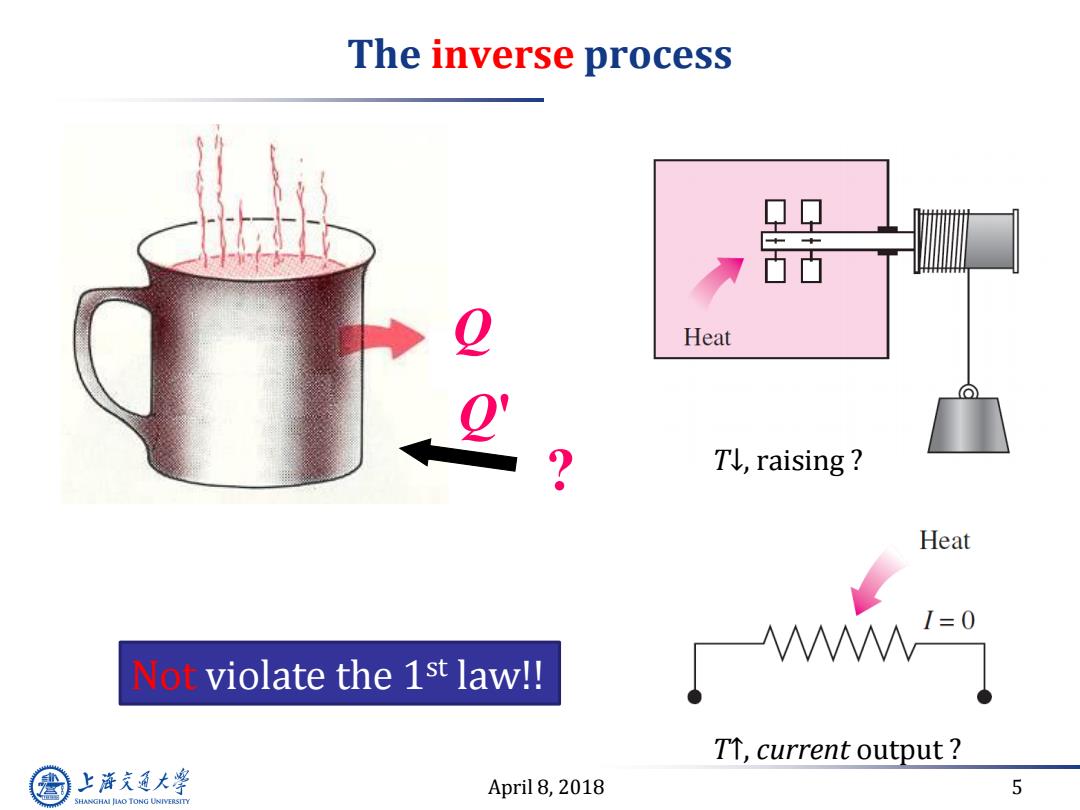
The inverse process Heat Tl,raising Heat Not violate the 1st law!! Iwm-9 TT,current output 上游充通大 April 8,2018 5 SHANGHAI JIAO TONG UNIVERSITY
April 8, 2018 5 The inverse process Q Q' ? Not violate the 1st law!! T↓, raising ? T↑, current output ?
Summary Spontaneous process: processes proceed in a certain direction initial condition of the system can be restored,but not in a spontaneous process.Some auxiliary devices would be required (a permanent change in the condition of the surroundings would result.) satisfying the first law does not ensure that the process can actually occur 上游充通大学 April 8,2018 6 SHANGHAI JLAO TONG UNIVERSITY
April 8, 2018 6 Summary Spontaneous process: • processes proceed in a certain direction • initial condition of the system can be restored, but not in a spontaneous process. Some auxiliary devices would be required (a permanent change in the condition of the surroundings would result.). • satisfying the first law does not ensure that the process can actually occur
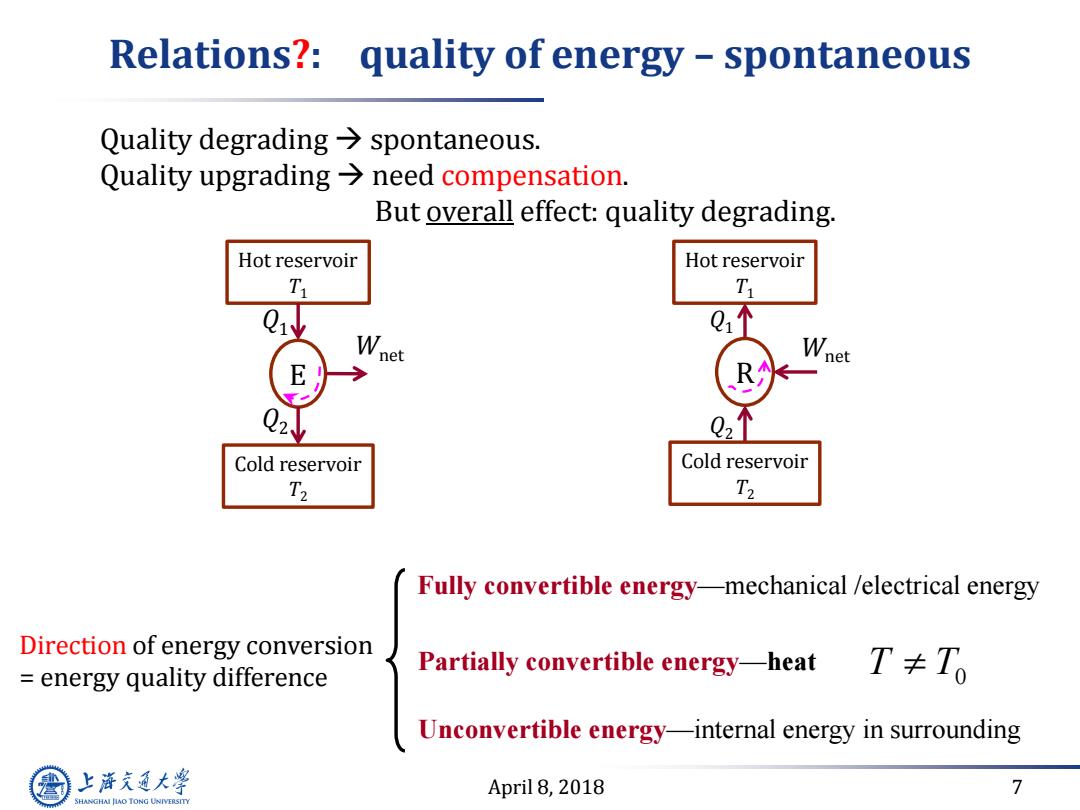
Relations?:quality of energy -spontaneous Quality degrading>spontaneous. Quality upgrading>need compensation. But overall effect:quality degrading. Hot reservoir Hot reservoir T T Wnet Wnet R 2 Q21 Cold reservoir Cold reservoir T2 T2 Fully convertible energy-mechanical /electrical energy Direction of energy conversion energy quality difference Partially convertible energy-heat T≠To Unconvertible energy-internal energy in surrounding 上游充通大 April 8,2018 7 SHANGHAI JIAO TONG UNIVERSITY
April 8, 2018 7 Relations?: quality of energy – spontaneous Quality degrading spontaneous. Quality upgrading need compensation. But overall effect: quality degrading. Hot reservoir T1 E Cold reservoir T2 Q1 Q2 Wnet Hot reservoir T1 R Cold reservoir T2 Q1 Q2 Wnet Direction of energy conversion = energy quality difference Fully convertible energy—mechanical /electrical energy Partially convertible energy—heat T T0 Unconvertible energy—internal energy in surrounding

Calling another principle-2nd law Only the first law is inadequate to identify whether a process can take place. Another guiding principle would be helpful.This is provided by the 2nd law The above inverse processes violate the 2nd law, so not spontaneous. A process cannot occur unless it satisfies both the first and the second laws of thermodynamics 上游充通大学 April 8,2018 8 SHANGHAI JLAO TONG UNIVERSITY
April 8, 2018 8 Calling another principle- 2 nd law Only the first law is inadequate to identify whether a process can take place. Another guiding principle would be helpful. This is provided by the 2nd law The above inverse processes violate the 2nd law, so not spontaneous. A process cannot occur unless it satisfies both the first and the second laws of thermodynamics
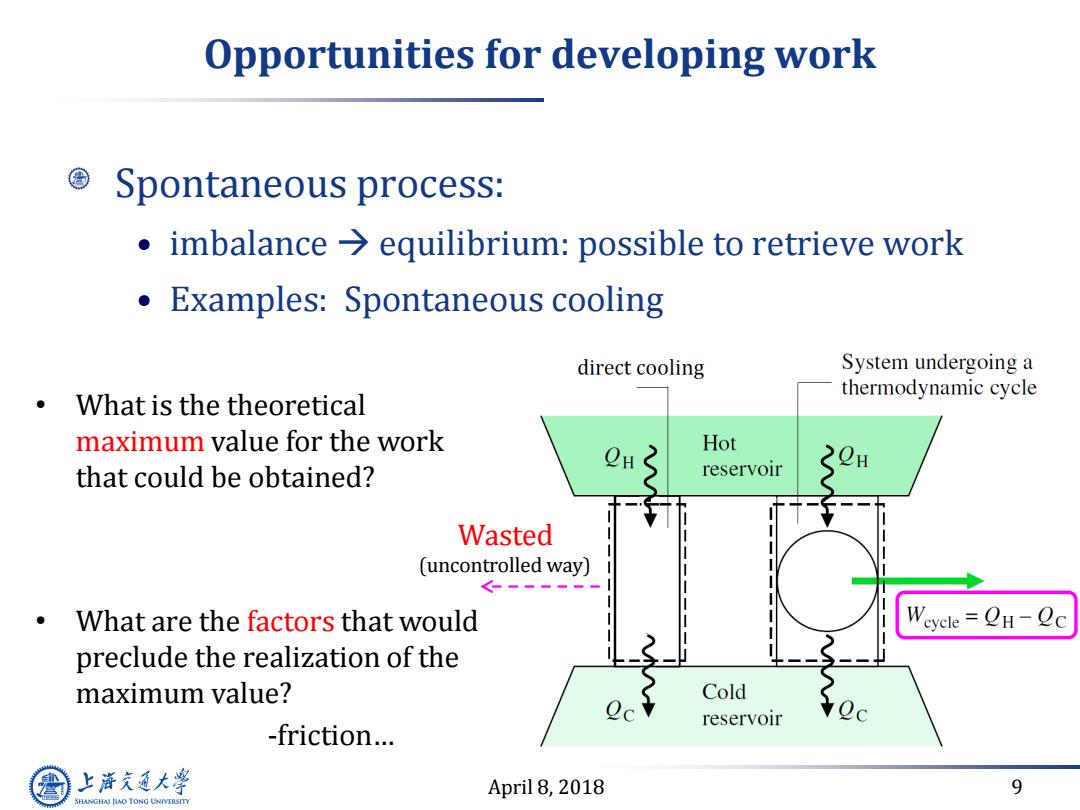
Opportunities for developing work Spontaneous process: imbalance equilibrium:possible to retrieve work Examples:Spontaneous cooling direct cooling System undergoing a thermodynamic cycle What is the theoretical maximum value for the work Hot that could be obtained? reservoir 20 Wasted (uncontrolled way) .What are the factors that would Weyele=CH-ec preclude the realization of the maximum value? Cold reservoir -friction... 上游充通大 April 8,2018 9 SHANGHAI JLAO TONG UNIVERSITY
April 8, 2018 9 Opportunities for developing work Spontaneous process: • imbalance equilibrium: possible to retrieve work • Examples: Spontaneous cooling Wasted (uncontrolled way) • What is the theoretical maximum value for the work that could be obtained? • What are the factors that would preclude the realization of the maximum value? -friction…
Summary:duty of the second law 1.predicting the direction of processes. 2.establishing conditions for equilibrium. 0 3.determining the best theoretical performance of cycles, engines,and other devices. 4.evaluating quantitatively the factors that preclude the attainment of the best theoretical performance level. >how to briefly and accurately state the 2nd law? 上游通大学 April 8,2018 10 HANGHAI JIAO TONG UNIVERSITY
April 8, 2018 10 Summary: duty of the second law 1. predicting the direction of processes. 2. establishing conditions for equilibrium. 3. determining the best theoretical performance of cycles, engines, and other devices. 4. evaluating quantitatively the factors that preclude the attainment of the best theoretical performance level. how to briefly and accurately state the 2nd law?