
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 37-38 Cengel Chapter 8 Exergy-A measure of work potential Spring,5/2/2018 Prof.,Dr.Yonghua HUANG 强 MAALLLMMA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 37-38 Spring, 5/2/2018 Prof., Dr. Yonghua HUANG Cengel Chapter 8 Exergy – A measure of work potential http://cc.sjtu.edu.cn/G2S/site/thermo.html
Previous:energy analysis All considered the isentropic process as the goal to strive for. Maximize isentropic efficiency This approach is short sighted for three reasons: 1.It ignores processes where heat transfer is present.(The majority of all practical processes.) 2.It assumes that reversibility can be obtained. 3.It assumes that the exit state of a device can "float",i.e.,cases where the exit pressure is fixed,but the exit temperature is allowed to fall below the temperature of the surroundings. >Need different approach for thermodynamic analysis: Exergy (Availability)Analysis 上游气通大 May2,2018 2 SHANGHAI JIAO TONG UNIVERSITY
May 2, 2018 2 Previous: energy analysis All considered the isentropic process as the goal to strive for. Maximize isentropic efficiency This approach is short sighted for three reasons: 1. It ignores processes where heat transfer is present.(The majority of all practical processes.) 2. It assumes that reversibility can be obtained. 3. It assumes that the exit state of a device can “float”, i.e., cases where the exit pressure is fixed, but the exit temperature is allowed to fall below the temperature of the surroundings. Need different approach for thermodynamic analysis: Exergy (Availability) Analysis
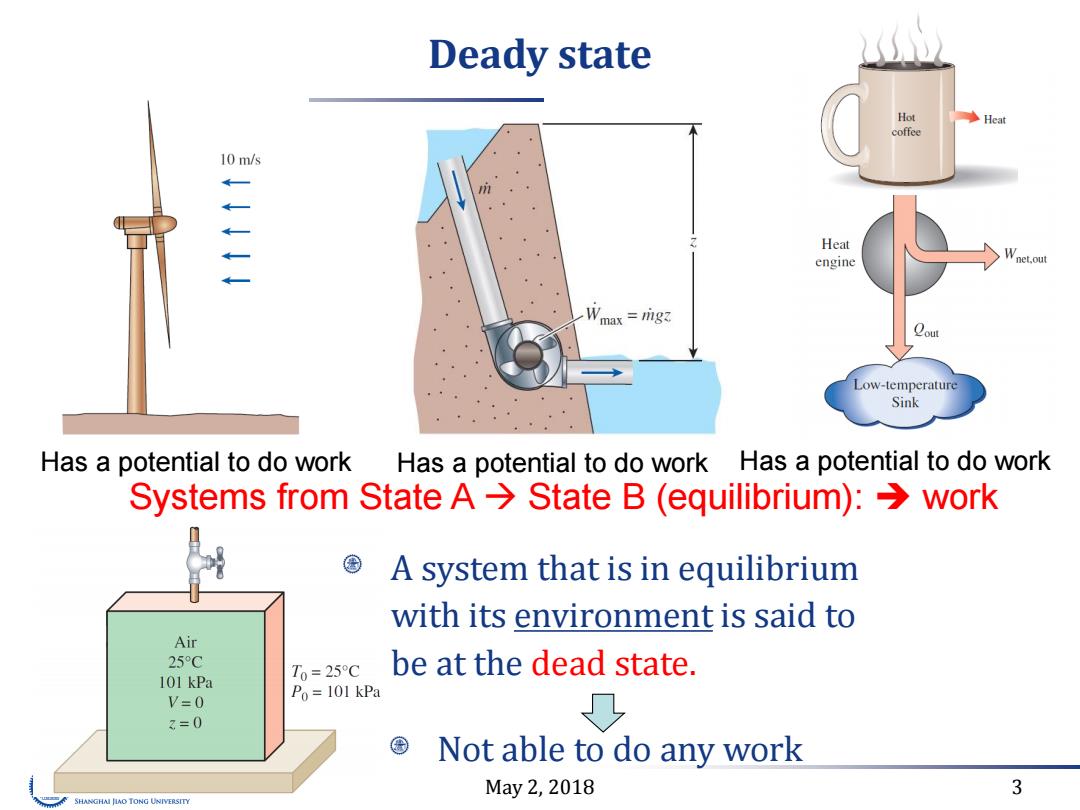
Deady state Hot ◆Heat coffee 10m/s Heat engine Wnet.out Wmax rig Low-temperature Sink Has a potential to do work Has a potential to do work Has a potential to do work Systems from State A>State B (equilibrium):>work A system that is in equilibrium with its environment is said to Air 25C 101 kPa T0=25C be at the dead state. V=0 Po=101 kPa 3=0 Not able to do any work May2,2018 3 SHANGHAI JIAO TONG UNIVERSITY
May 2, 2018 3 Deady state A system that is in equilibrium with its environment is said to be at the dead state. Has a potential to do work Has a potential to do work Has a potential to do work Not able to do any work Systems from State A State B (equilibrium): work
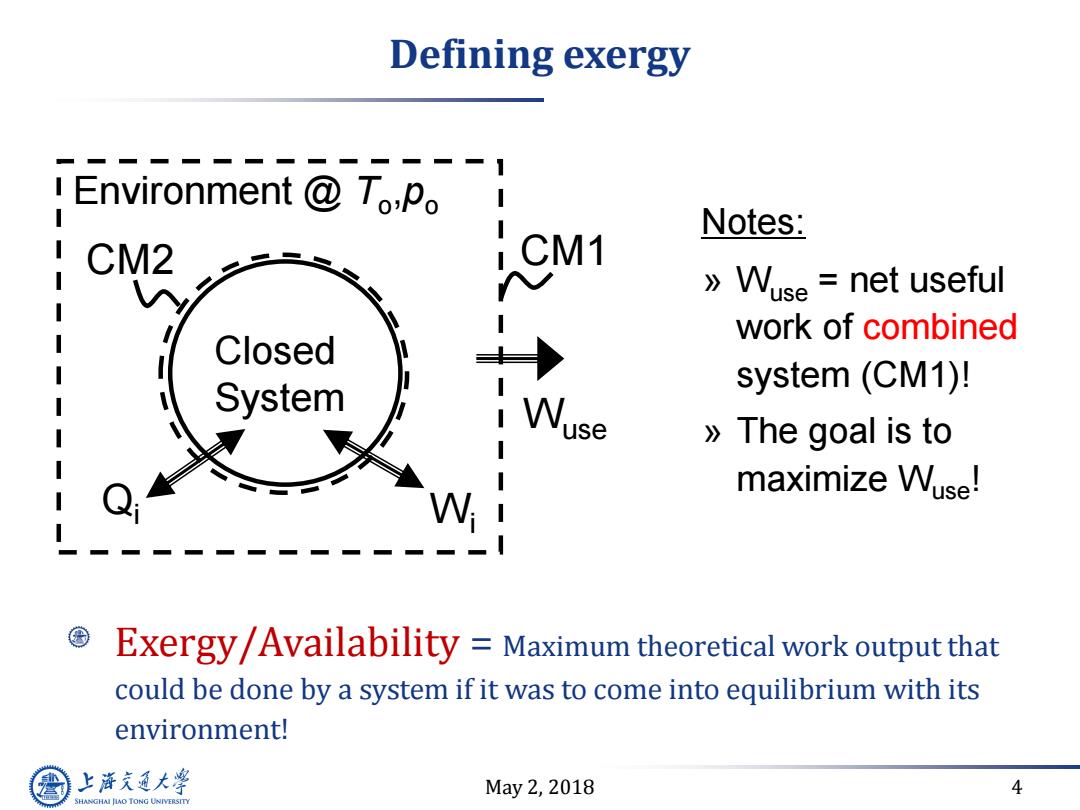
Defining exergy Environment To:Po Notes: CM2 CM1 》Wuse=net useful work of combined I Closed I System system(CM1)! use 》The goal is to maximize Wuse! Exergy/Availability Maximum theoretical work output that could be done by a system if it was to come into equilibrium with its environment! 上游充通大 May2,2018 4 SHANGHAI JLAO TONG UNIVERSITY
May 2, 2018 4 Defining exergy Exergy/Availability = Maximum theoretical work output that could be done by a system if it was to come into equilibrium with its environment! Notes: » Wuse = net useful work of combined system (CM1)! » The goal is to maximize Wuse! Closed System Environment @ To ,po Qi CM2 CM1 Wi Wuse
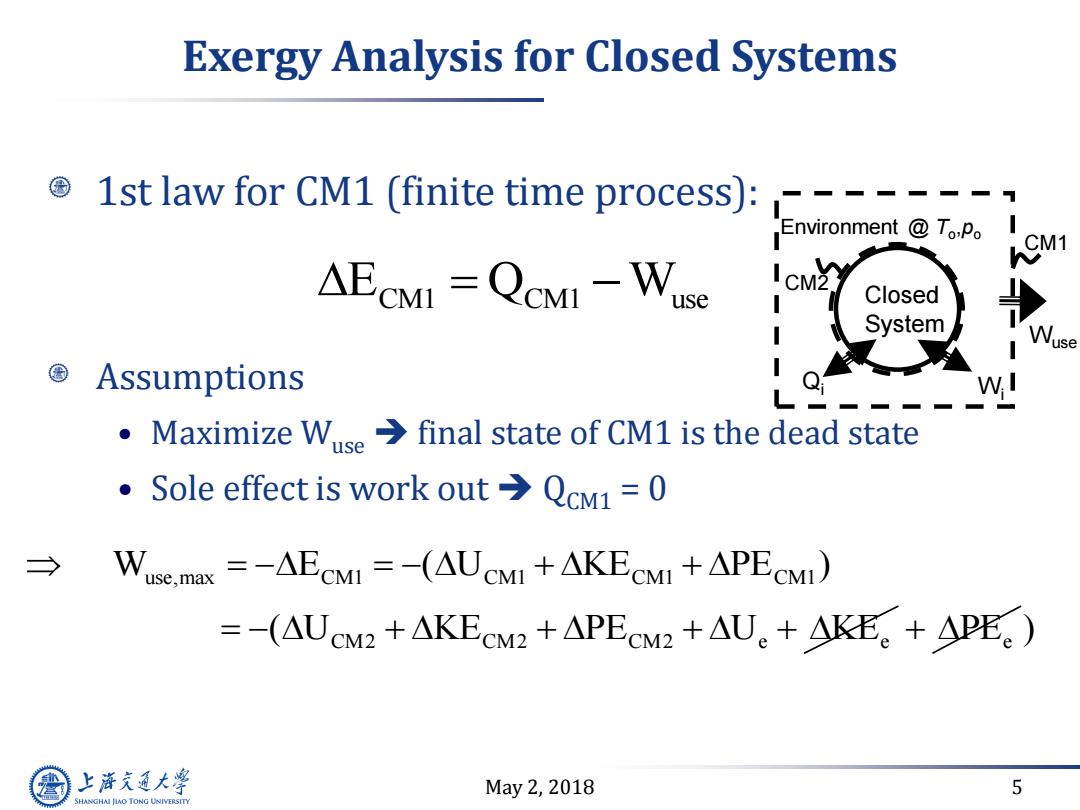
Exergy Analysis for Closed Systems © 1st law for CM1 (finite time process):---- Environment To.Po AECMI =QCMI -Wuse Closed System I Wuse Assumptions Maximize Wuse>final state of CM1 is the dead state e Sole effect is work out>QcM1=0 → Wuse.max =-AECMI=-(AUCMI +AKECMI +APECMI) =-(AUCM2+AKECM22+APECM2+AU。+KE。+APEe) 上游气通大粤 May2,2018 5 SHANGHAI JLAO TONG UNIVERSITY
May 2, 2018 5 Exergy Analysis for Closed Systems 1st law for CM1 (finite time process): Assumptions • Maximize Wuse final state of CM1 is the dead state • Sole effect is work out QCM1 = 0 E Q W CM1 CM1 use use,max CM1 CM1 CM1 CM1 CM2 CM2 CM2 e e W E ( U KE PE ) ( U KE PE U KE PEe ) Closed System Environment @ To ,po Qi CM2 CM1 Wi Wuse
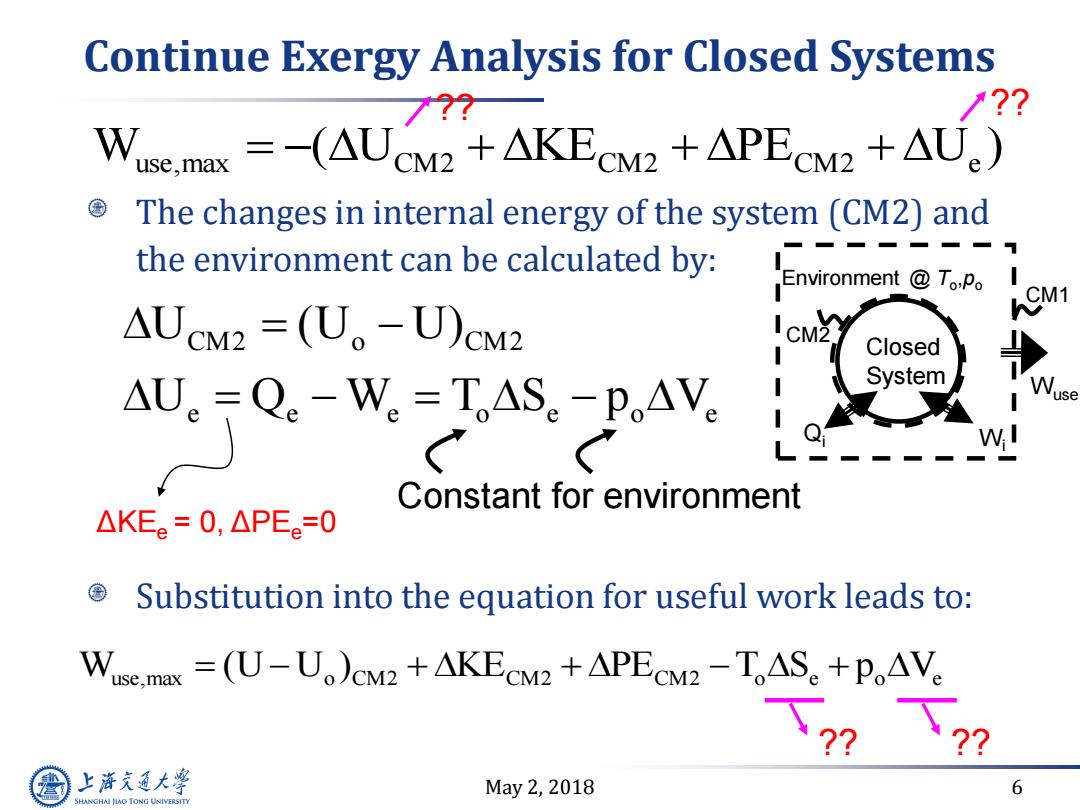
Continue Exergy Analysis for Closed Systems 77? 1?? Wuse.max =-(AUCM2 +AKECM2 +APECM2 +AU) The changes in internal energy of the system(CM2)and the environment can be calculated by: .Environment ToPo AUcM2=(U。-U)cM2 I CM2 Closed AU.Q。-W,=IAS.-P,AV System Constant for environment △KEe=0,△PEe=0 Substitution into the equation for useful work leads to: Wuse.masx =(U-U)CM2 +AKECM2+APECM2-TAS +PoAVe 上游通大学 May2,2018 6 SHANGHAI JIAO TONG UNIVERSITY
May 2, 2018 6 Continue Exergy Analysis for Closed Systems The changes in internal energy of the system (CM2) and the environment can be calculated by: Substitution into the equation for useful work leads to: CM2 o CM2 e e e o e o e U (U U) U Q W T S p V Constant for environment W (U U ) KE PE T S p V use,max o CM2 CM2 CM2 o e o e Closed System Environment @ To ,po Qi CM2 CM1 Wi Wuse W ( U KE PE U ) use,max CM2 CM2 CM2 e ?? ?? ?? ?? ΔKEe = 0, ΔPEe=0
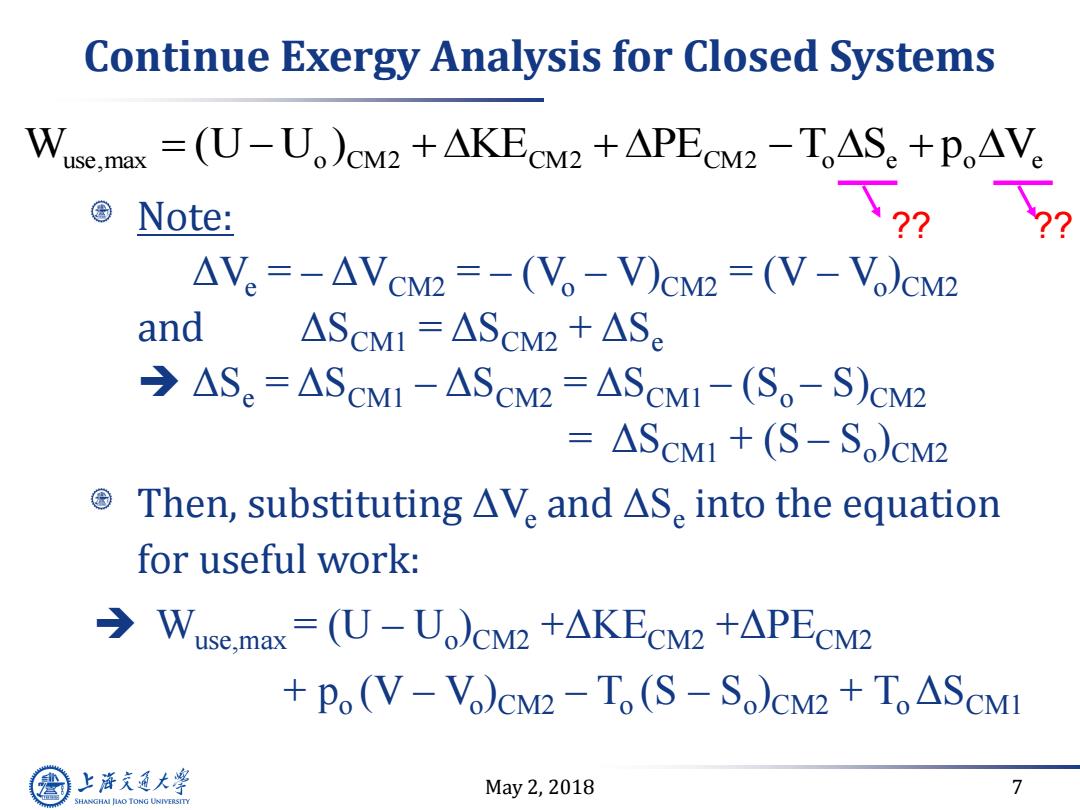
Continue Exergy Analysis for Closed Systems Wuse.max =(U-U)CM2 +AKECM2 +APECM2-TAS+PoAVe Note: ?? △V。=-AVcM2=-(V。-V)cM2=(V-V)cM2 and ASCmI AScM2 ASe →ASe=AScMI-AScM2=AScM1-(S。-S)CM2 =△SCM1+(S-S)cM2 Then,substituting△V。and△S.into the equation for useful work: Wuse.max=(U-U)CM2 +AKECM2+APECM2 Po(V-Vo)CM2-To(S-So)CM2 ToASCMI 上游充通大学 May2,2018 7 SHANGHAI JLAO TONG UNIVERSITY
May 2, 2018 7 Continue Exergy Analysis for Closed Systems Note: ΔVe = – ΔVCM2 = – (Vo – V)CM2 = (V – Vo )CM2 and ΔSCM1 = ΔSCM2 + ΔSe ΔSe = ΔSCM1 – ΔSCM2 = ΔSCM1 – (So – S)CM2 = ΔSCM1 + (S – So )CM2 Then, substituting ΔVe and ΔSe into the equation for useful work: Wuse,max = (U – Uo )CM2 +ΔKECM2 +ΔPECM2 + po (V – Vo )CM2 – To (S – So )CM2 + To ΔSCM1 W (U U ) KE PE T S p V use,max o CM2 CM2 CM2 o e o e ?? ??
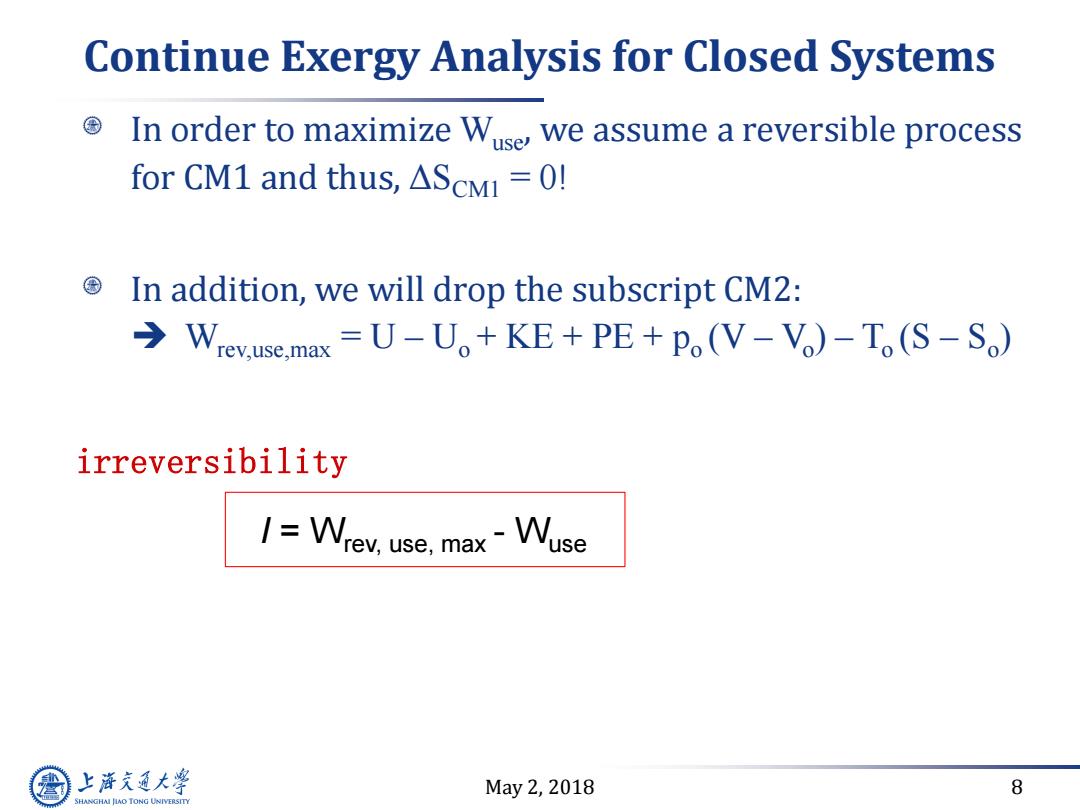
Continue Exergy Analysis for Closed Systems In order to maximize Wuse,we assume a reversible process for CM1 and thus,AScMi=0! In addition,we will drop the subscript CM2: Wrev.use.max =U-Uo+KE+PE+po(V-V)-To(S-So) irreversibility I=Wrev,use,max-Wuse 上游充通大学 May2,2018 8 SHANGHAI JLAO TONG UNIVERSITY
May 2, 2018 8 Continue Exergy Analysis for Closed Systems In order to maximize Wuse, we assume a reversible process for CM1 and thus, ΔSCM1 = 0! In addition, we will drop the subscript CM2: Wrev,use,max = U – Uo + KE + PE + po (V – Vo ) – To (S – So ) irreversibility I = Wrev, use, max - Wuse
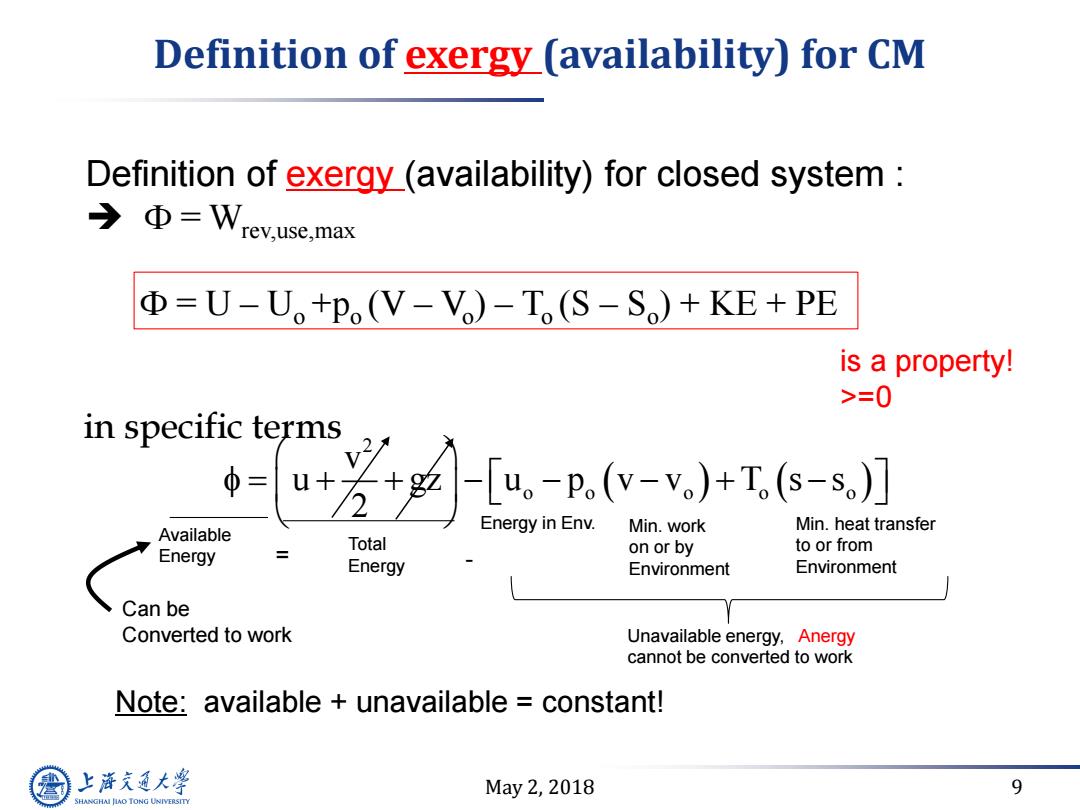
Definition of exergy(availability)for CM Definition of exergy_(availability)for closed system →Φ=Wrev.use,.max Φ=U-U。+p(V-Vo)-T,(S-So)+KE+PE is a property! >=0 in specific terms 芳-e-)T- = Available Energy in Env. Min.work Min.heat transfer Total to or from Energy on or by Energy Environment Environment Can be Converted to work Unavailable energy,Anergy cannot be converted to work Note:available unavailable constant! 上游充通大 May2,2018 9 SHANGHAI JIAO TONG UNIVERSITY
May 2, 2018 9 Definition of exergy (availability) for CM 2 o o o o o v u gz u p v v T s s 2 Note: available + unavailable = constant! Available Energy Min. work on or by Environment Min. heat transfer to or from Environment Total Energy = - Can be Converted to work Unavailable energy, Anergy cannot be converted to work in specific terms Energy in Env. Definition of exergy (availability) for closed system : F = Wrev,use,max F = U – Uo +po (V – Vo ) – To (S – So ) + KE + PE is a property! >=0
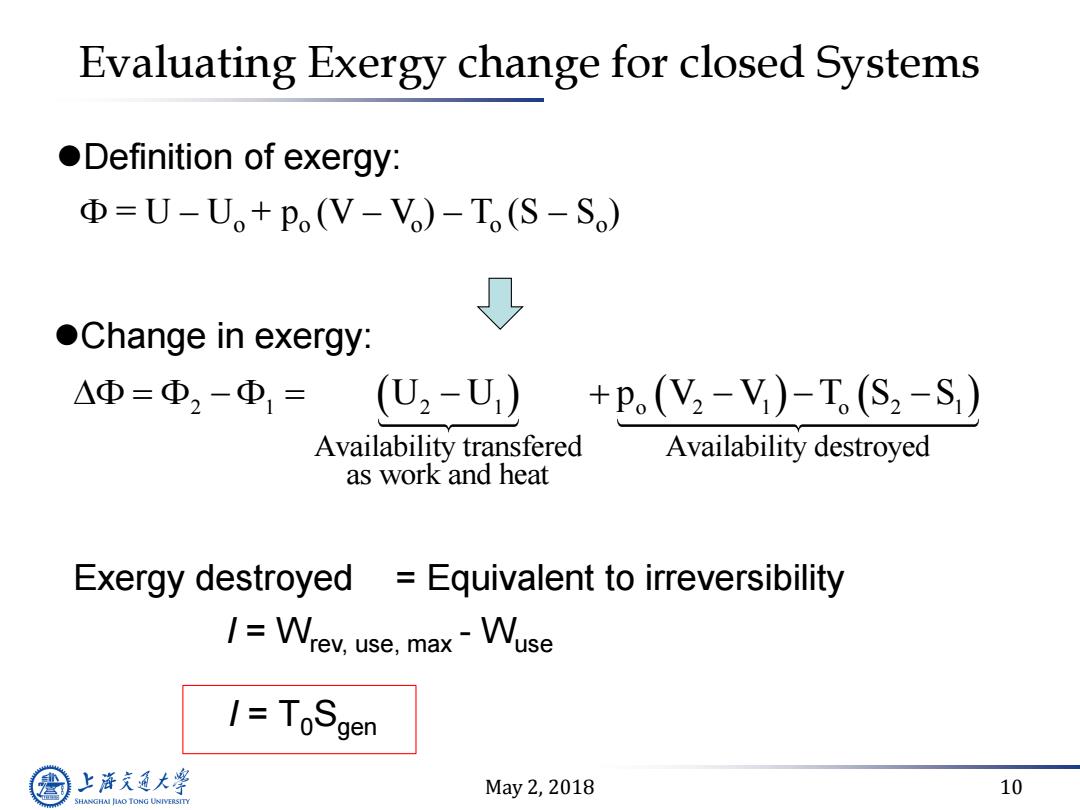
Evaluating Exergy change for closed Systems oDefinition of exergy: D=U-U+po(V-V)-To(S-So) ↓ Change in exergy: △Φ=Φ2-Φ1=( U2-U) +P(V2-V)-T(S2-S) Availability transfered Availability destroyed as work and heat Exergy destroyed Equivalent to irreversibility 1=Wrev,use,max-Wuse I=ToSgen 上游充通大 May2,2018 10 SHANGHAI JLAO TONG UNIVERSITY
May 2, 2018 10 Evaluating Exergy change for closed Systems Change in exergy: 2 1 2 1 o 2 1 o 2 1 Availability transfered Availability destroyed as work and heat F F F U U p V V T S S F = U – Uo + po (V – Vo ) – To (S – So ) Definition of exergy: Exergy destroyed I = Wrev, use, max - Wuse = Equivalent to irreversibility I = T0Sgen