上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 11 Spring,3/14/2019 福 Prof.,Dr.Yonghua HUANG VLLMMAA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日G
Engineering Thermodynamics I Lecture 11 Spring, 3/14/2019 Prof., Dr. Yonghua HUANG http://cc.sjtu.edu.cn/G2S/site/thermo.html
Retrieving thermodynamic properties Tables Important skill Graphs How? RT a Equations py=RT p - A四gwm/naE■ Computer software 1T1100 (EES,IT,Refprop...) Pejb-a) 1100002 E171网 http://www.cryo.sjtu.edu.cn/cryofluid/ 上游充通大 March 14,2019 2 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 2 Retrieving thermodynamic properties How? Tables Graphs Equations Computer software (EES, IT, Refprop…) Important skill pv RT http://www.cryo.sjtu.edu.cn/cryofluid/
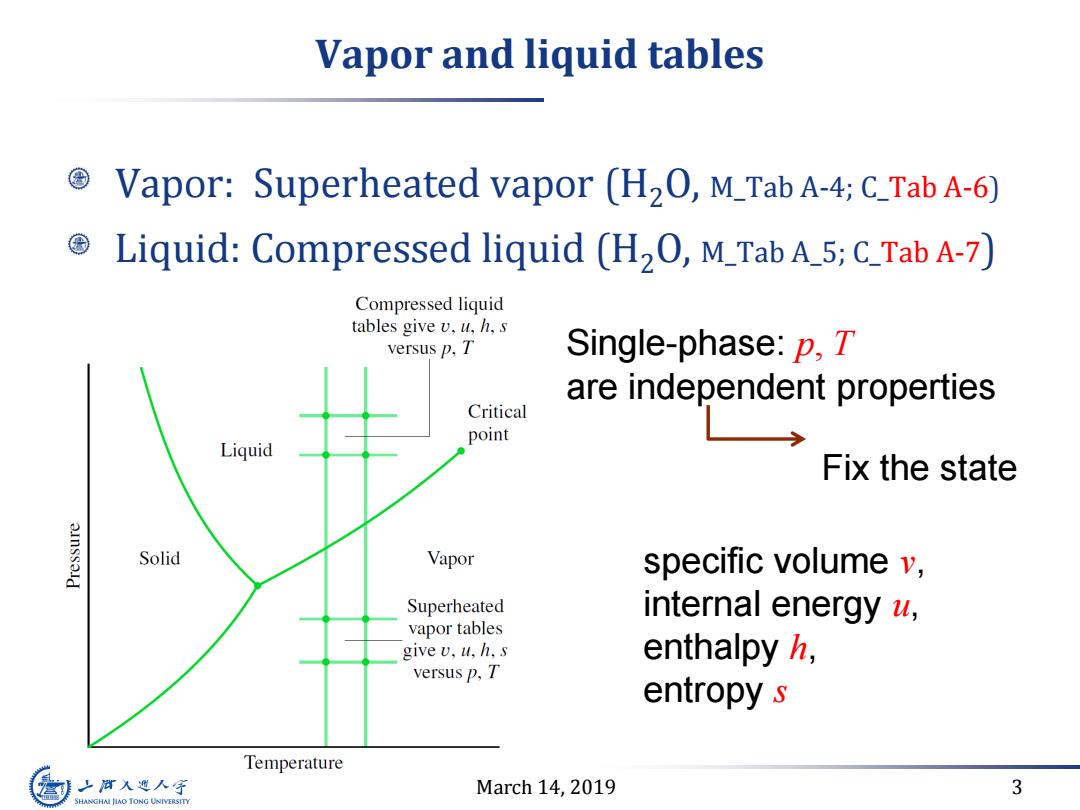
Vapor and liquid tables Vapor:Superheated vapor (H2O,M_Tab A-4;C_Tab A-6) Liquid:Compressed liquid (H2O,M_Tab A_5;C_Tab A-7) Compressed liquid tables give v,u,h,s versus p,T Single-phase:p,T are independent properties Critical point Liquid Fix the state Solid Vapor specific volume v, Superheated internal energy a, vapor tables give v,u,h,s enthalpy h, versus p,T entropy s Temperature 之酒入避人字 March 14,2019 3 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 3 Vapor and liquid tables Vapor: Superheated vapor (H2O, M_Tab A-4; C_Tab A-6) Liquid: Compressed liquid (H2O, M_Tab A_5; C_Tab A-7) Single-phase: p, T are independent properties Fix the state specific volume v, internal energy u, enthalpy h, entropy s
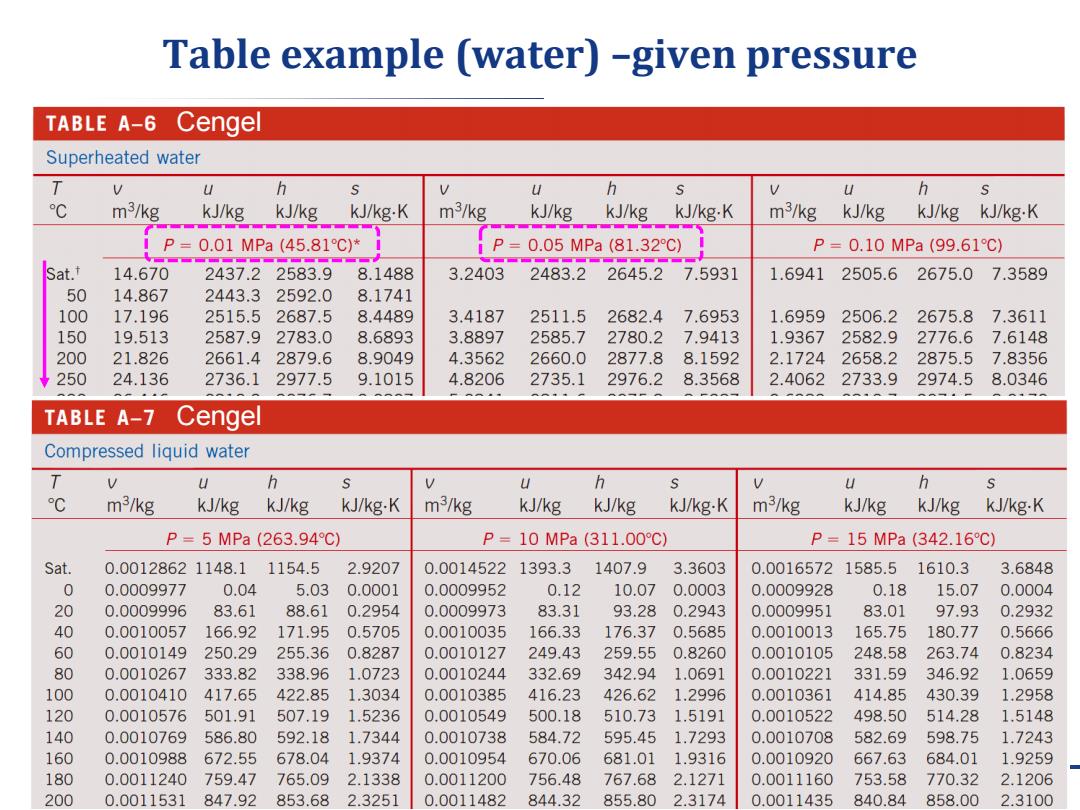
Table example (water)-given pressure TABLE A-6 Cengel Superheated water V u h h U h m3/kg kJ/kg kJ/kg kJ/kg.K m3/kg kJ/kg kJ/kg kJ/kg-K m3/kg kJ/kg kJ/kg kJ/kg.K P=0.01MPa(45.81℃)*i P= 0.05MPa(81.32C) P=0.10MPa(99.61℃) at. 14.670 2437.22583.9 8.1488 3.2403 2483.2 2645.2 7.5931 1.6941 2505.6 2675.0 7.3589 50 14.867 2443.3 2592.0 8.1741 100 17.196 2515.5 2687.5 8.4489 3.4187 2511.5 2682.4 7.6953 1.6959 2506.2 2675.8 7.3611 150 19.513 2587.9 2783.0 8.6893 3.8897 2585.7 2780.2 7.9413 1.9367 2582.9 2776.67.6148 200 21.826 2661.4 2879.6 8.9049 4.3562 2660.0 2877.8 8.1592 2.1724 2658.2 2875.5 7.8356 250 24.136 2736.1 2977.5 9.1015 4.8206 2735.1 2976.2 8.3568 2.4062 2733.9 2974.5 8.0346 TABLE A-7 Cengel Compressed liquid water T V U h U h U h ℃ m3/kg kJ/kg kJ/kg kJ/kg-K m3/kg kJ/kg kJ/kg kJ/kg-K m3/kg kJ/kg kJ/kg kJ/kg-K P=5MPa(263.94℃) P=10MPa(311.00℃) P=15MPa(342.16C) Sat. 0.00128621148.1 1154.5 2.9207 0.0014522 1393.3 1407.9 3.3603 0.0016572 1585.5 1610.3 3.6848 0 0.0009977 0.04 5.03 0.0001 0.0009952 0.12 10.07 0.0003 0.0009928 0.18 15.07 0.0004 20 0.0009996 83.61 88.61 0.2954 0.0009973 83.31 93.28 0.2943 0.0009951 83.01 97.93 0.2932 40 0.0010057 166.92 171.95 0.5705 0.0010035 166.33 176.37 0.5685 0.0010013 165.75 180.77 0.5666 60 0.0010149 250.29 255.36 0.8287 0.0010127 249.43 259.55 0.8260 0.0010105 248.58 263.74 0.8234 80 0.0010267 333.82 338.96 1.0723 0.0010244 332.69 342.94 1.0691 0.0010221 331.59 346.92 1.0659 100 0.0010410 417.65 422.85 1.3034 0.0010385 416.23 426.62 1.2996 0.0010361 414.85 430.39 1.2958 120 0.0010576 501.91 507.19 1.5236 0.0010549 500.18 510.73 1.5191 0.0010522 498.50 514.28 1.5148 140 0.0010769 586.80 592.18 1.7344 0.0010738 584.72 595.45 1.7293 0.0010708 582.69 598.75 1.7243 160 0.0010988 672.55 678.04 1.9374 0.0010954 670.06 681.01 1.9316 0.0010920 667.63 684.01 1.9259 180 0.0011240 759.47 765.09 2.1338 0.0011200 756.48 767.68 2.1271 0.0011160 753.58 770.32 2.1206 200 0.0011531 847.92 853.68 2.3251 0.0011482 844.32 855.80 2.3174 0.0011435 840.84 858.00 2.3100
March 14, 2019 4 Table example (water) –given pressure Cengel Cengel

Linear Interpolation Assumes any two data points connected by straight line (set slopes equal to find missing value) 240C,0.2275 p=10 bar ke T(°C) v (m/kg) 200 0.2060 (215C,v) 215 0=? 240 0.2275 200°C,0.2060 215-200 v-0.2060 200 215 240 240-100 0.2275-0.2060 TC) v=0.2141m3/kg 上游充通大学 March 14,2019 5 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 5 Linear Interpolation Assumes any two data points connected by straight line (set slopes equal to find missing value) 215 200 0.2060 240 100 0.2275 0.2060 v
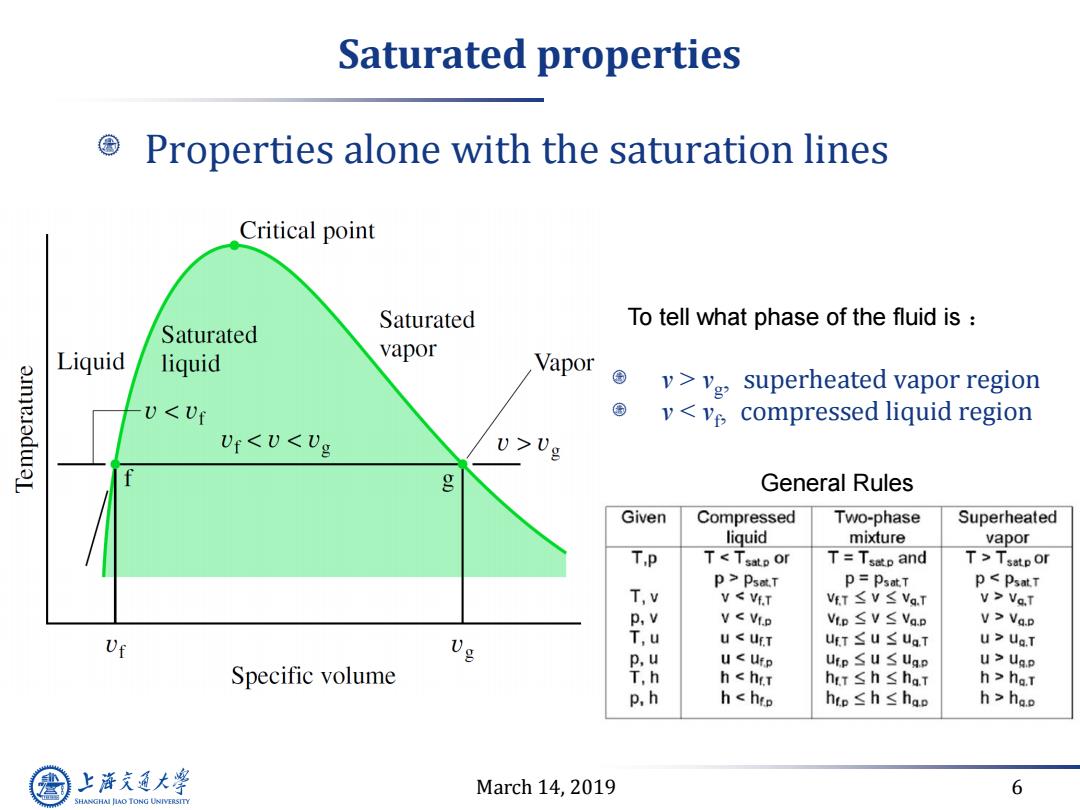
Saturated properties Properties alone with the saturation lines Critical point Saturated To tell what phase of the fluid is Saturated Liquid liquid vapor Vapor a.ImeladwaL v>vg,superheated vapor region UUg 1 g General Rules Given Compressed Two-phase Superheated liquid mixture vapor TP TTsatp or p>Psot.T p=Psat.T pVa.T p,v VVa.p Uf 0% T,u U Uf.T uT≤u≤uaT U>Ua.T p,u u Ufp uip≤u S Ugp u>Ug.p Specific volume T,h hha.T p,h hhao 上游充通大 March 14,2019 6 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 6 Saturated properties Properties alone with the saturation lines v > vg , superheated vapor region v < vf , compressed liquid region General Rules To tell what phase of the fluid is :
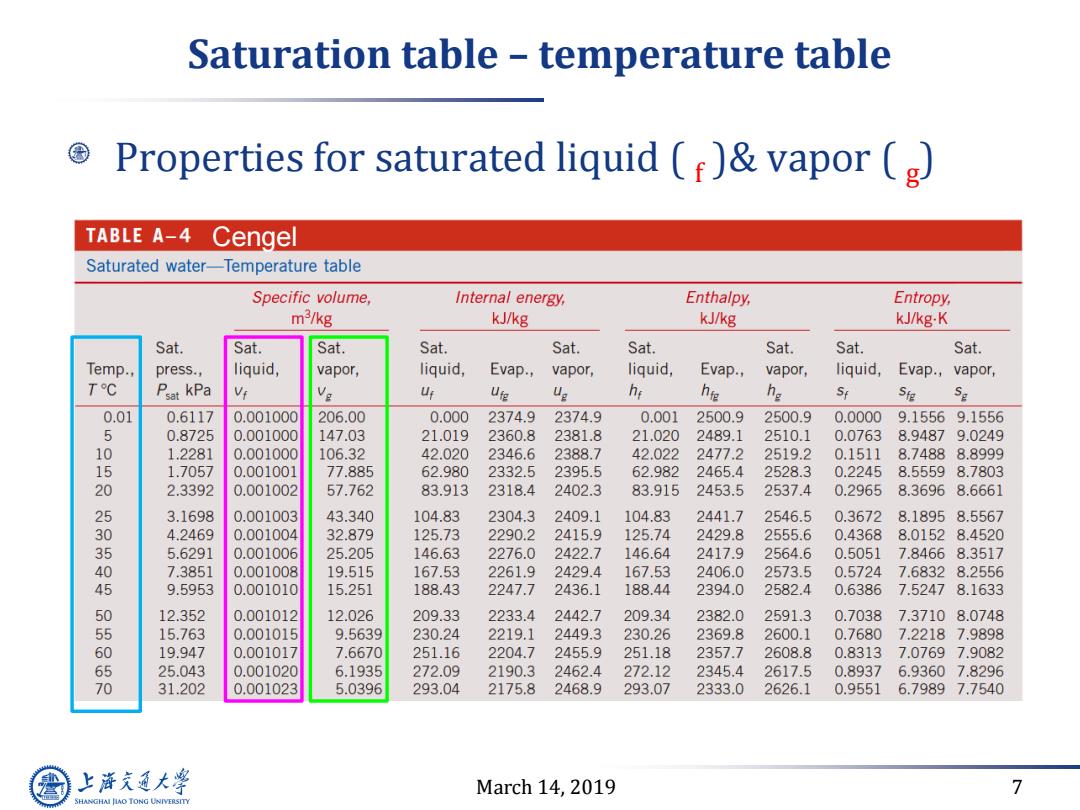
Saturation table temperature table Properties for saturated liquid ()vapor (g) TABLE A-4 Cenge Saturated water-Temperature table Specific volume, Internal energy, Enthalpy, Entropy, m3/kg kJ/kg kJ/kg kJ/kg.K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Temp., press., liquid, vapor, liquid, Evap., vapor, liquid, Evap., vapor, liquid, Evap.,vapor, TC Psat kPa U Ufg V2 he h移 he St Sig 0.01 0.6117 0.001000 206.00 0.000 2374.9 2374.9 0.001 2500.9 2500.9 0.0000 9.15569.1556 6 0.8725 0.001000 147.03 21.019 2360.8 2381.8 21.020 2489.1 2510.1 0.0763 8.94879.0249 10 1.2281 0.001000 106.32 42.020 2346.6 2388.7 42.022 2477.2 2519.2 0.1511 8.74888.8999 15 1.7057 0.001001 77.885 62.980 2332.5 2395.5 62.982 2465.4 2528.3 0.2245 8.55598.7803 20 2.3392 0.001002 57.762 83.913 2318.4 2402.3 83.915 2453.5 2537.4 0.2965 8.36968.6661 25 3.1698 0.001003 43.340 104.83 2304.3 2409.1 104.83 2441.7 2546.5 0.3672 8.18958.5567 30 4.2469 0.001004 32.879 125.73 2290.2 2415.9 125.74 2429.8 2555.6 0.4368 8.01528.4520 35 5.6291 0.001006 25.205 146.63 2276.0 2422.7 146.64 2417.9 2564.6 0.5051 7.84668.3517 40 7.3851 0.001008 19.515 167.53 2261.9 2429.4 167.53 2406.0 2573.5 0.5724 7.68328.2556 45 9.5953 0.001010 15.251 188.43 2247.7 2436.1 188.44 2394.0 2582.4 0.6386 7.52478.1633 50 12.352 0.001012 12.026 209.33 2233.4 2442.7 209.34 2382.0 2591.3 0.7038 7.37108.0748 5 15.763 0.001015 9.5639 230.24 2219.1 2449.3 230.26 2369.8 2600.1 0.7680 7.22187.9898 19.947 0.001017 7.6670 251.16 2204.7 2455.9 251.18 2357.7 2608.8 0.83137.0769 7.9082 25.043 0.001020 6.1935 272.09 2190.3 2462.4 272.12 2345.4 2617.5 0.8937 6.93607.8296 70 31.202 0.001023 5.0396 293.04 2175.8 2468.9 293.07 2333.0 2626.1 0.9551 6.79897.7540 上降充通大学 March 14,2019 7 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 7 Saturation table – temperature table Properties for saturated liquid ( f )& vapor ( g ) Cengel
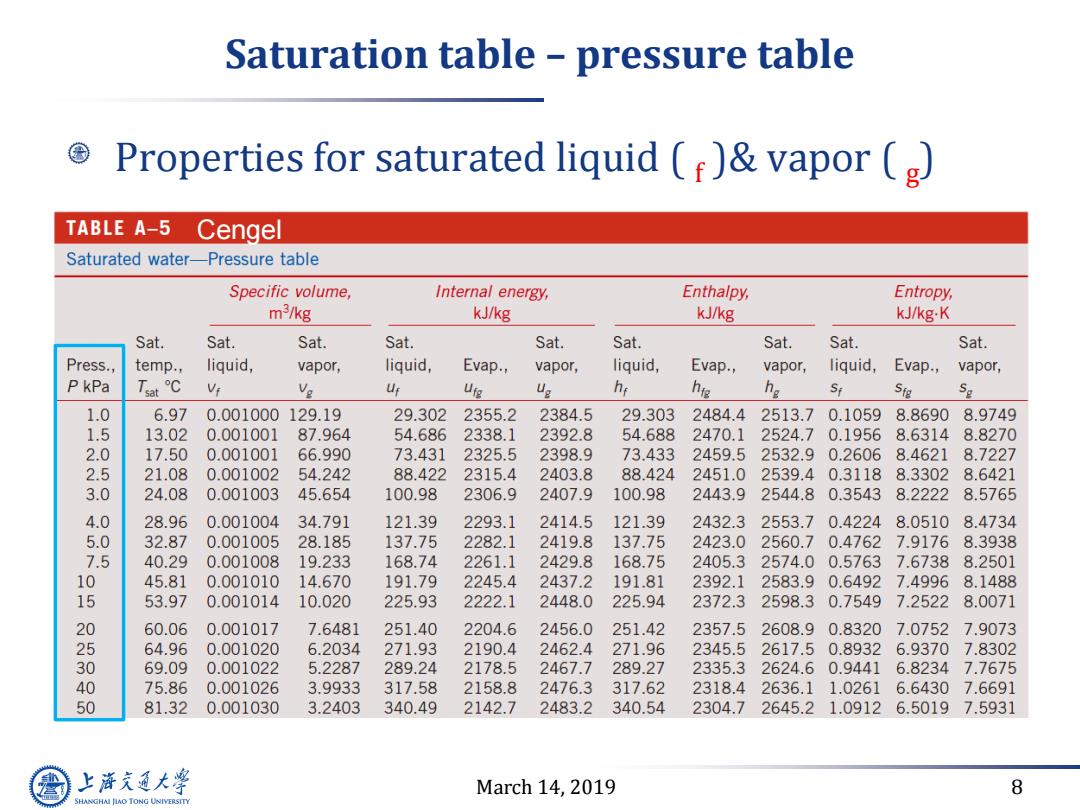
Saturation table -pressure table Properties for saturated liquid ()vapor (g) TABLE A-5 Cengel Saturated water-Pressure table Specific volume, Internal energy, Enthalpy, Entropy, m3/kg kJ/kg kJ/kg kJ/kg.K Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Sat. Press., temp., liquid, vapor, liquid, Evap., vapor, liquid, Evap., vapor, liquid, Evap., vapor, P kPa Tat℃ Ve Uf Urg g hr hie % St Sig Sg 1.0 6.97 0.001000129.19 29.302 2355.2 2384.5 29.303 2484.4 2513.7 0.1059 8.8690 8.9749 1.5 13.02 0.001001 87.964 54.686 2338.1 2392.8 54.688 2470.1 2524.70.1956 8.6314 8.8270 2.0 17.50 0.001001 66.990 73.431 2325.5 2398.9 73.433 2459.5 2532.90.2606 8.4621 8.7227 2.5 21.08 0.001002 54.242 88.422 2315.4 2403.8 88.424 2451.0 2539.40.3118 8.3302 8.6421 3.0 24.08 0.001003 45.654 100.98 2306.9 2407.9 100.98 2443.9 2544.80.3543 8.2222 8.5765 4.0 28.96 0.001004 34.791 121.39 2293.1 2414.5 121.39 2432.32553.70.4224 8.0510 8.4734 5.0 32.87 0.001005 28.185 137.75 2282.1 2419.8 137.75 2423.0 2560.70.4762 7.9176 8.3938 7.5 40.29 0.001008 19.233 168.74 2261.1 2429.8 168.75 2405.32574.00.5763 7.6738 8.2501 10 45.81 0.001010 14.670 191.79 2245.4 2437.2 191.81 2392.12583.90.6492 7.49968.1488 15 53.97 0.001014 10.020 225.93 2222.1 2448.0 225.94 2372.3 2598.30.7549 7.2522 8.0071 20 60.06 0.001017 7.6481 251.40 2204.6 2456.0 251.42 2357.52608.90.8320 7.0752 7.9073 25 64.96 0.001020 6.2034 271.93 2190.4 2462.4 271.96 2345.52617.50.89326.9370 7.8302 04050 69.09 0.001022 5.2287 289.24 2178.5 2467.7 289.27 2335.32624.60.9441 6.82347.7675 75.86 0.001026 3.9933 317.58 2158.8 2476.3 317.62 2318.42636.11.0261 6.6430 7.6691 81.32 0.001030 3.2403 340.49 2142.7 2483.2 340.54 2304.7 2645.2 1.0912 6.5019 7.5931 上降充通大学 March 14,2019 8 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 8 Saturation table – pressure table Properties for saturated liquid ( f )& vapor ( g ) Cengel
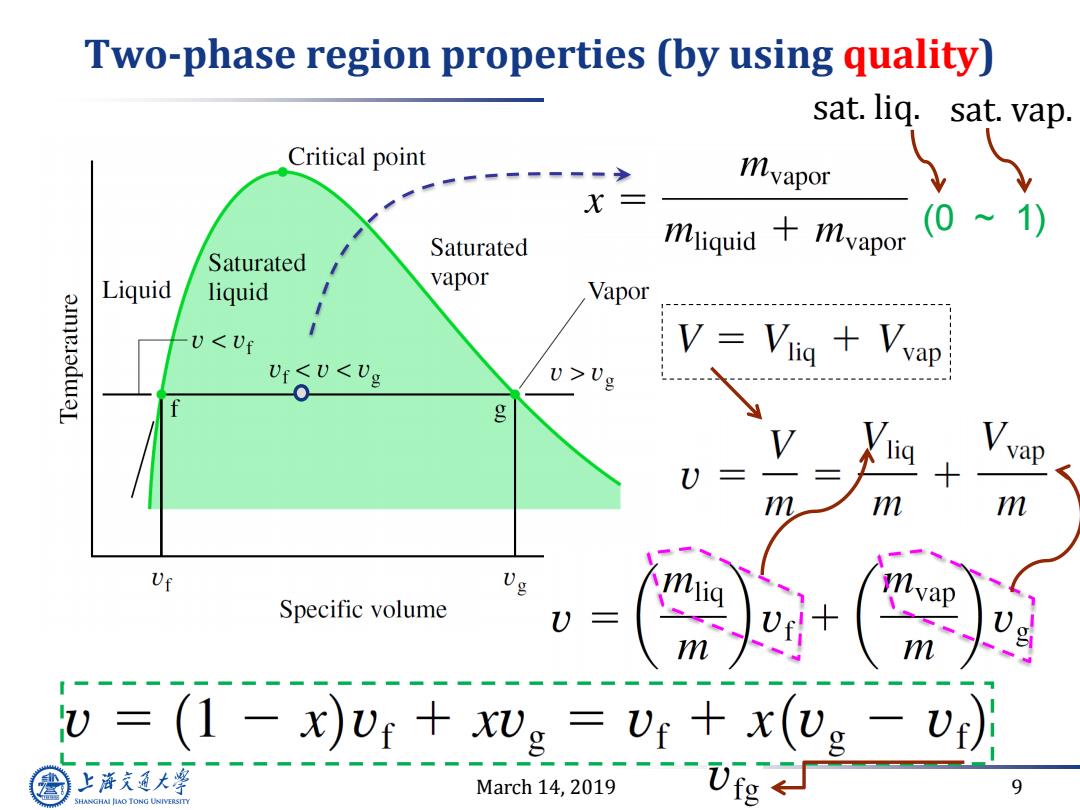
Two-phase region properties (by using quality) sat.liq.sat.vap. Critical point myapor X三 mliquid+mvapor (0~ 1 Saturated Saturated Liquid liquid vapor Vapor 3.mmeledwaL UUg g m m Uf Specific volume vap m m =(1-x)Ur+xvg=Ur+x(vg-vr) 上本夫通大等 March 14,2019 9 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 9 Two-phase region properties (by using quality) (0 ~ 1) sat. liq. sat. vap
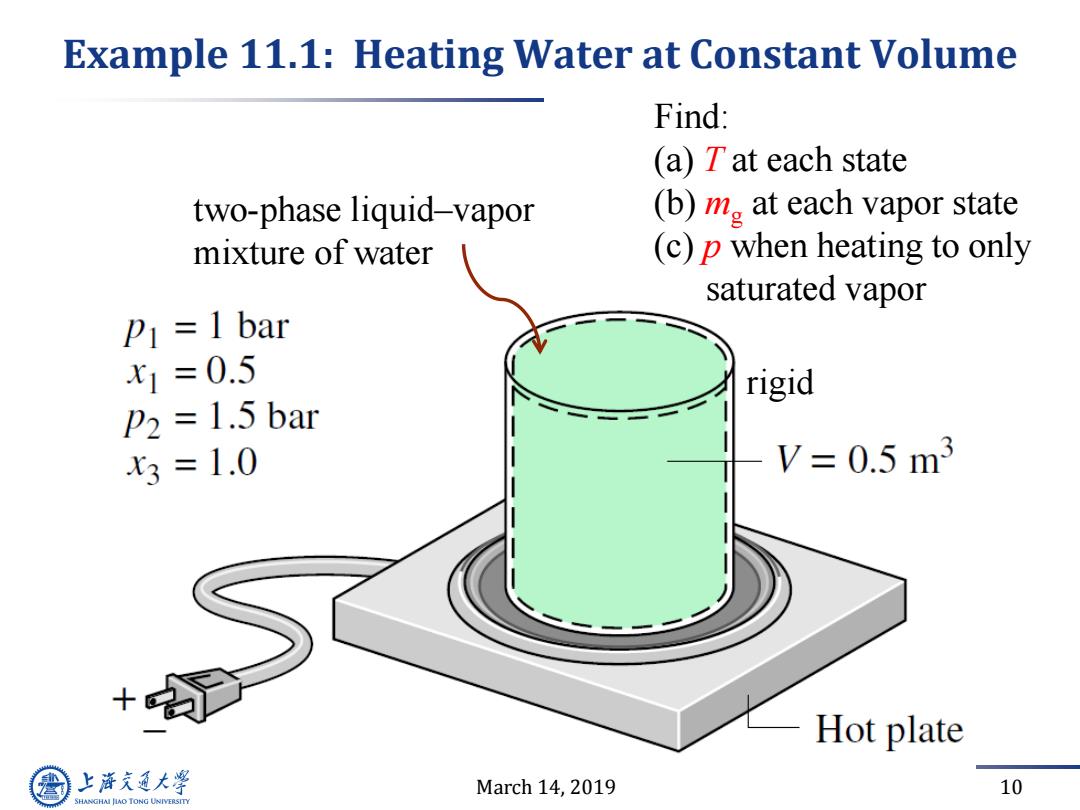
Example 11.1:Heating Water at Constant Volume Find: (a)Tat each state two-phase liquid-vapor (b)m at each vapor state mixture of water (c)p when heating to only saturated vapor P1 =1 bar x1=0.5 rigid P2 =1.5 bar x3=1.0 V=0.5m3 Hot plate 上游究通大学 March 14,2019 10 SHANGHAI JIAO TONG UNIVERSITY
March 14, 2019 10 Example 11.1: Heating Water at Constant Volume Find: (a) T at each state (b) mg at each vapor state (c) p when heating to only saturated vapor two-phase liquid–vapor mixture of water rigid