上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 36 Chapter 7 Entropy (Section7.10-7.11) Spring,4/28/2019 漏 Prof.,Dr.Yonghua HUANG AA http://cc.sjtu.edu.cn/G2S/site/thermo.html
Engineering Thermodynamics I Lecture 36 Spring, 4/28/2019 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.10-7.11) http://cc.sjtu.edu.cn/G2S/site/thermo.html
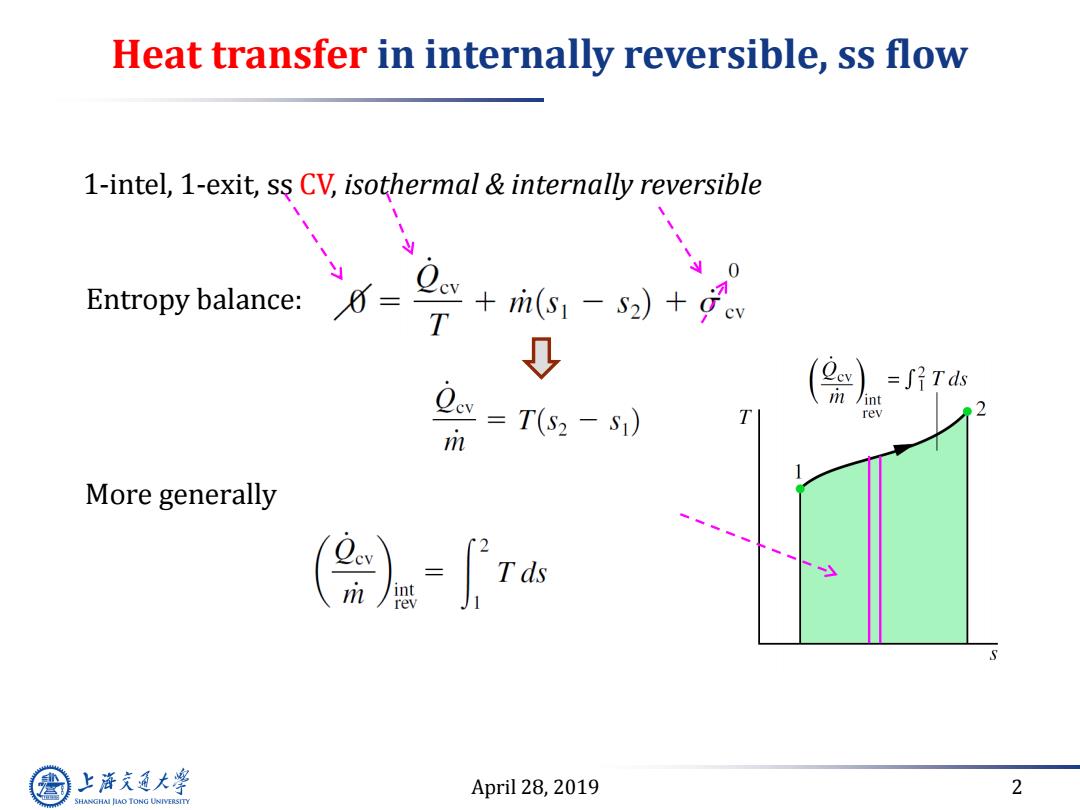
Heat transfer in internally reversible,ss flow 1-intel,1-exit,ss CV,isothermal internally reversible y Entropy balance: +i6,-s)+。 T 0 =∫?Tds w=T(s-s) int rev m More generally ()= 上游究通大学 April 28,2019 2 SHANGHAI JLAO TONG UNIVERSITY
April 28, 2019 2 Heat transfer in internally reversible, ss flow 1-intel, 1-exit, ss CV, isothermal & internally reversible More generally Entropy balance:

Work in internal reversible,ss flow 1-intel,1-exit,ss CV,isothermal internally reversible ss,energy balance: -品价+)+ m Internally reversible! m 2nd entropy eq.Tds =dh-v dp T=-)- vdp =0 for nozzles/diffusers 0 下、(Bernoulli eq, ?-V3 J斤vdp +g(31-2) C W,in Chinese textbook (Often neglected) Technical work-the mechanical energy can be applied directly 上游充通大 April 28,2019 3 SHANGHAI JIAO TONG UNIVERSITY
April 28, 2019 3 Work in internal reversible, ss flow ss, energy balance: 1-intel, 1-exit, ss CV, isothermal & internally reversible Internally reversible 2 nd entropy eq. (Often neglected) Technical work- the mechanical energy can be applied directly Wt in Chinese textbook =0 for nozzles/diffusers (Bernoulli eq.)
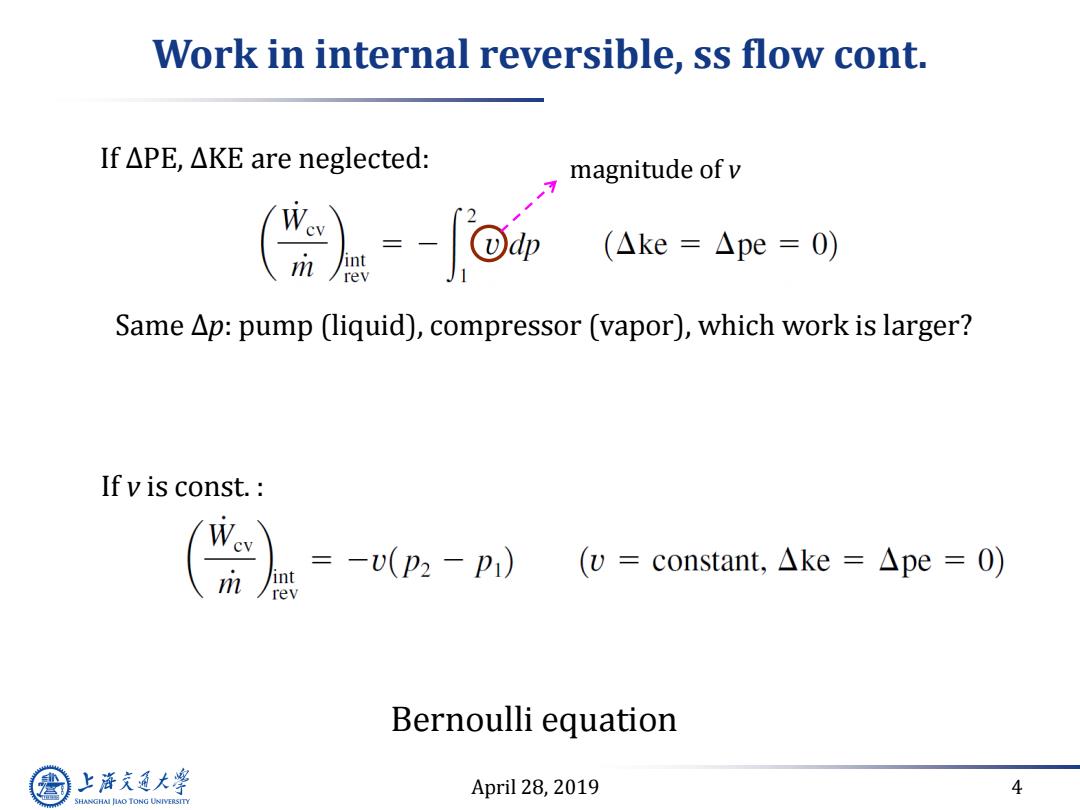
Work in internal reversible,ss flow cont. If△PE,△KE are neglected: magnitude of v (Ake=△pe=0) Same Ap:pump (liquid),compressor (vapor),which work is larger? If v is const. m =-v(p2-p1) int (v=constant,.△ke=△pe=O) rev Bernoulli equation 上游究通大学 April 28,2019 4 SHANGHAI JLAO TONG UNIVERSITY
April 28, 2019 4 Work in internal reversible, ss flow cont. Bernoulli equation If ∆PE, ∆KE are neglected: If v is const. : Same ∆p: pump (liquid), compressor (vapor), which work is larger? magnitude of v

Work in polytropic process,reversible,ss Polytropic:pu"constant If△PE,△KE are neglected: -=-omm -1 m rev p2U2-p1U1) (polytropic,n≠l) Any gas/liquid Different from the one for closed system,eq.(3.54) w=y-p出 (n≠1) 1-n =-(p1w)ln(p2/p1) (polytropic,n 1) 上降文通大学 April 28,2019 5 SHANGHAI JIAO TONG UNIVERSITY
April 28, 2019 5 Work in polytropic process, reversible, ss Polytropic: If ∆PE, ∆KE are neglected: Any gas/liquid Different from the one for closed system, eq.(3.54) 2 2 1 1 (n 1) 1 p v p v w n

Work in polytropic process,reversible,ss,Ideal gas Ideal gas:pv=RT M m /int (polytropic,n≠1) rev nR int n-1 (T-T) (ideal gas,n≠1) rev (ideal gas,n≠1) =-(p心)lnp/p) int (polytropic,n 1) rev (ideal gas,n 1) int =-RTIn(p2/p1) rev 上游充通大 April 28,2019 6 SHANGHAI JIAO TONG UNIVERSITY
April 28, 2019 6 Work in polytropic process, reversible, ss, Ideal gas Ideal gas: pv=RT or
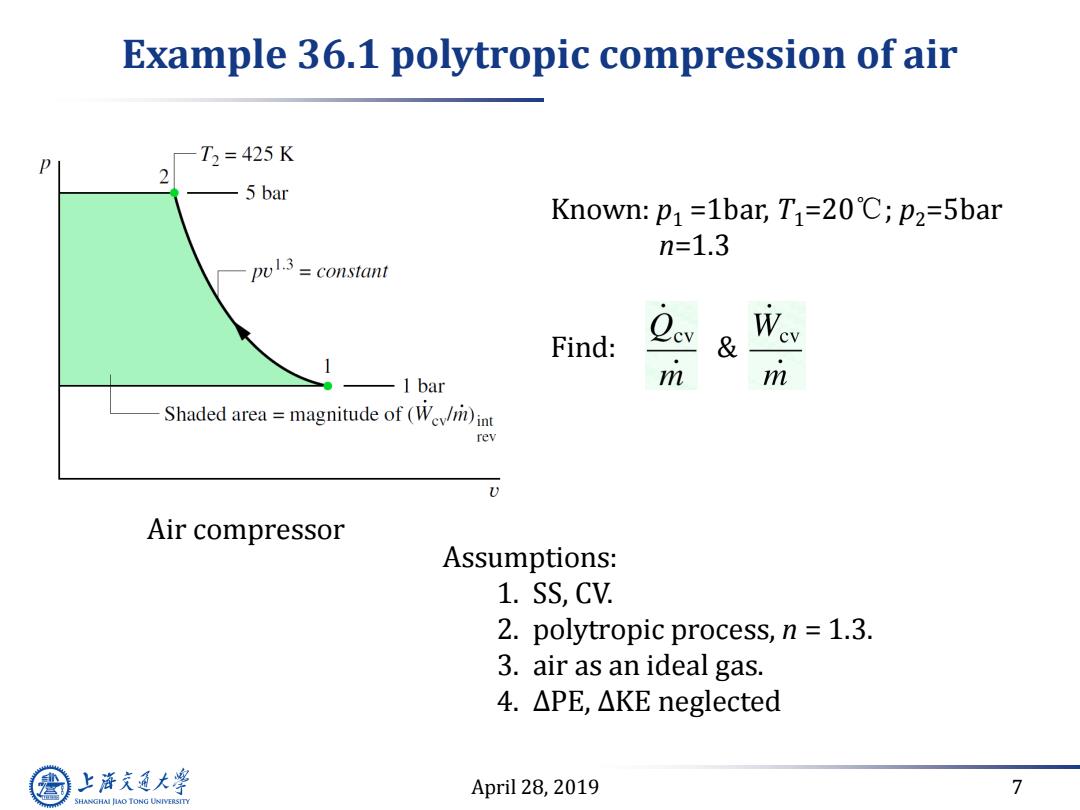
Example 36.1 polytropic compression of air T2=425K 2 5bar Known:p1=1bar,T1=20C;p2=5bar n=1.3 pu3=constant Find: & -1 bar m m Shaded area=magnitude of (We)in rev Air compressor Assumptions: 1.SS,CV. 2.polytropic process,n =1.3. 3.air as an ideal gas. 4.△PE,△KE neglected 上游充通大学 April 28,2019 7 SHANGHAI JIAO TONG UNIVERSITY
April 28, 2019 7 Example 36.1 polytropic compression of air Known: p1 =1bar, T1=20℃; p2=5bar n=1.3 Find: & Assumptions: 1. SS, CV. 2. polytropic process, n = 1.3. 3. air as an ideal gas. 4. ∆PE, ∆KE neglected Air compressor
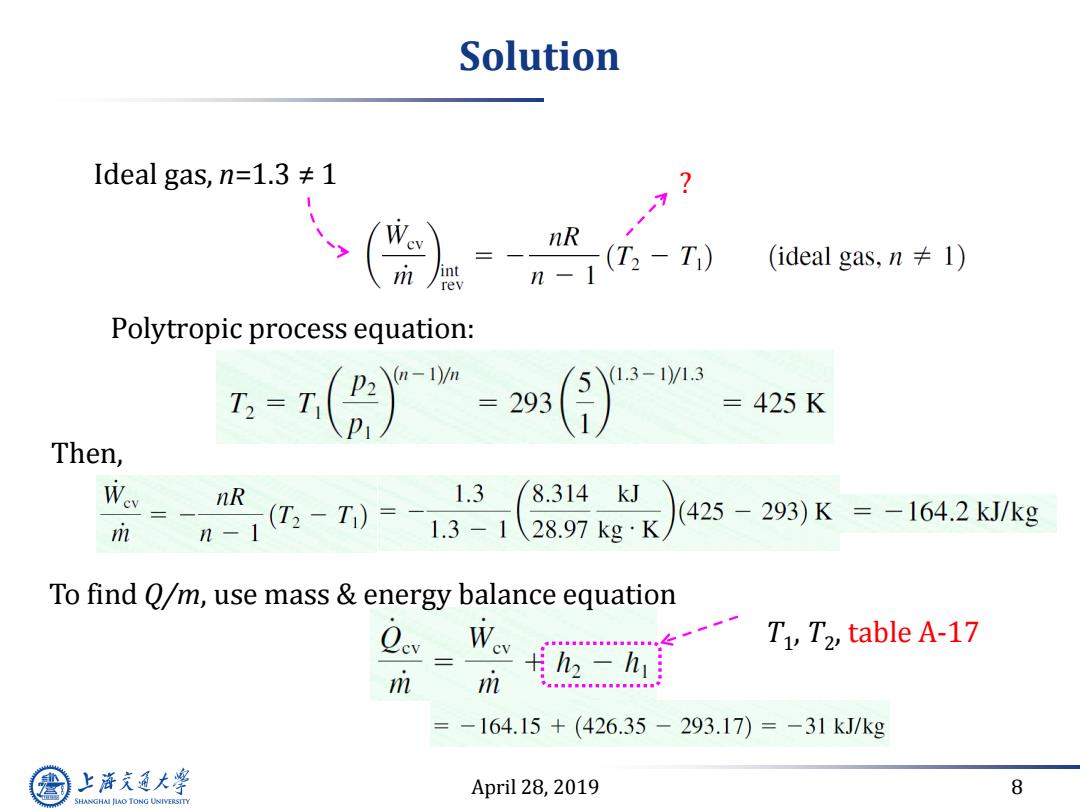
Solution Ideal gas,n=1.3≠1 nR 12 -T) (ideal gas,.n≠l) rev Polytropic process equation: (1.3-1)/1.3 T=T( =293 =425K Then, Wev nR 1.3 8.314kJ i n=-T)= 1.3-1 28.97kg·K (425-293)K=-164.2kJ/kg To find Q/m,use mass energy balance equation Ccv Wev -T1,T2,table A-17 m m h2-h =-164.15+(426.35-293.17))=-31kJ/kg 上游充通大 April 28,2019 8 SHANGHAI JIAO TONG UNIVERSITY
April 28, 2019 8 Solution Ideal gas, n=1.3 ≠ 1 ? Polytropic process equation: Then, To find Q/m, use mass & energy balance equation T1 , T2 , table A-17
Homework: Problems:M 6.155 上游充通大学 April 28,2019 9 SHANGHAI JLAO TONG UNIVERSITY
April 28, 2019 9 Homework: Problems: M_6.155