
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 38 Cengel Chapter 8 Exergy-A measure of work potential Spring,4/30/2019 Prof.,Dr.Yonghua HUANG 强 MAMMMAAN http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 38 Spring, 4/30/2019 Prof., Dr. Yonghua HUANG Cengel Chapter 8 Exergy – A measure of work potential http://cc.sjtu.edu.cn/G2S/site/thermo.html
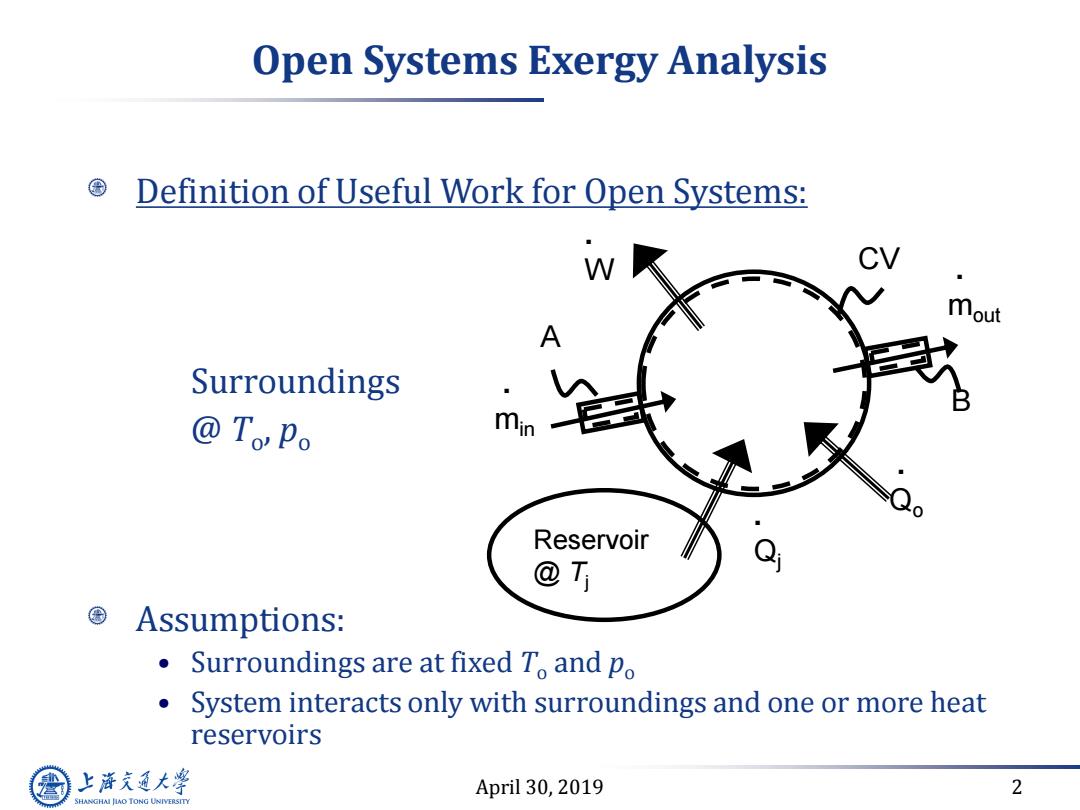
Open Systems Exergy Analysis Definition of Useful Work for Open Systems: W mout Surroundings @ToPo Reservoir @T Assumptions: ● Surroundings are at fixed To and po System interacts only with surroundings and one or more heat reservoirs 上游充通大 April 30,2019 2 SHANGHAI JLAO TONG UNIVERSITY
April 30, 2019 2 Open Systems Exergy Analysis Definition of Useful Work for Open Systems: Surroundings @ To , po Assumptions: • Surroundings are at fixed To and po • System interacts only with surroundings and one or more heat reservoirs . W . Qj . min . mout B A CV . Qo Reservoir @ Tj
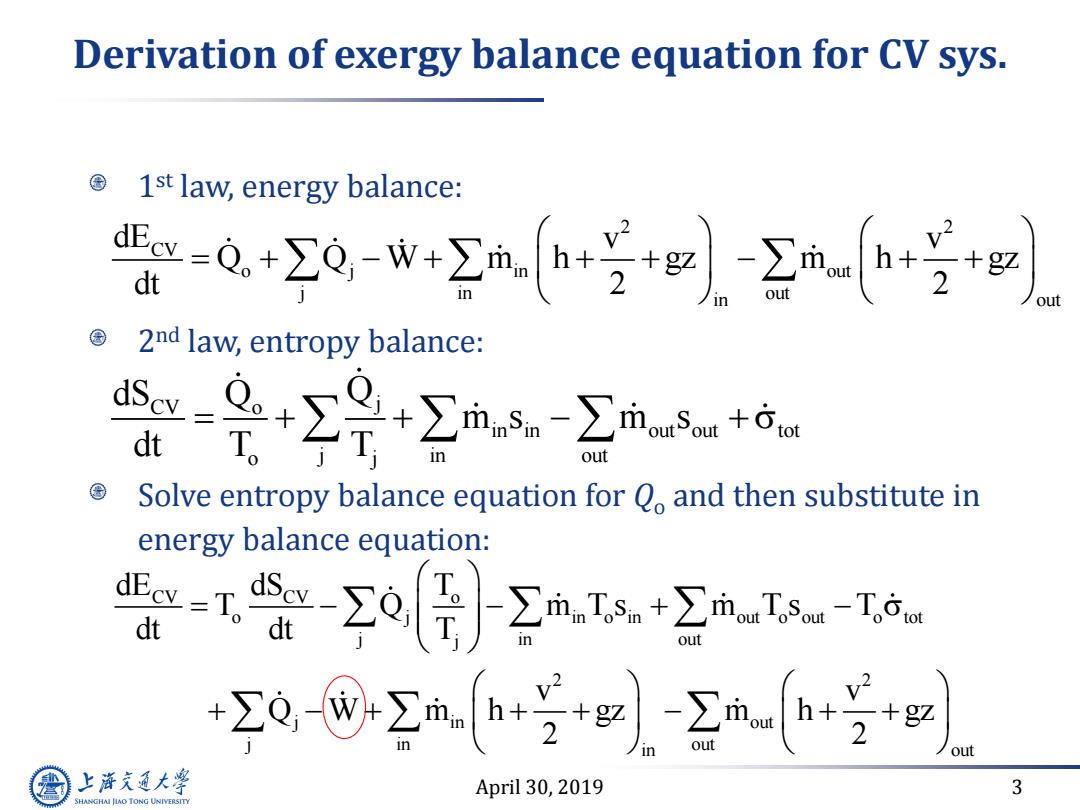
Derivation of exergy balance equation for CV sys. 1st law,energy balance: -0,w号 dt out 2nd law,entropy balance: -是+∑是+∑msm+ dt T。 out Solve entropy balance equation for Q,and then substitute in energy balance equation: -工o[aIs+2n- dt out +0-8mh++-2m+ +g2 out 2 out 上游通大学 April 30,2019 3 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 3 Derivation of exergy balance equation for CV sys. 1 st law, energy balance: 2 nd law, entropy balance: Solve entropy balance equation for Qo and then substitute in energy balance equation: 2 2 CV o j in out j in out in out dE v v Q Q W m h gz m h gz dt 2 2 CV o j in in out out tot o j j in out dS Q Q m s m s dt T T CV CV o o j in o in out o out o tot j in out j 2 2 j in out j in out in out dE dS T T Q m T s m T s T dt dt T v v Q W m h gz m h gz 2 2
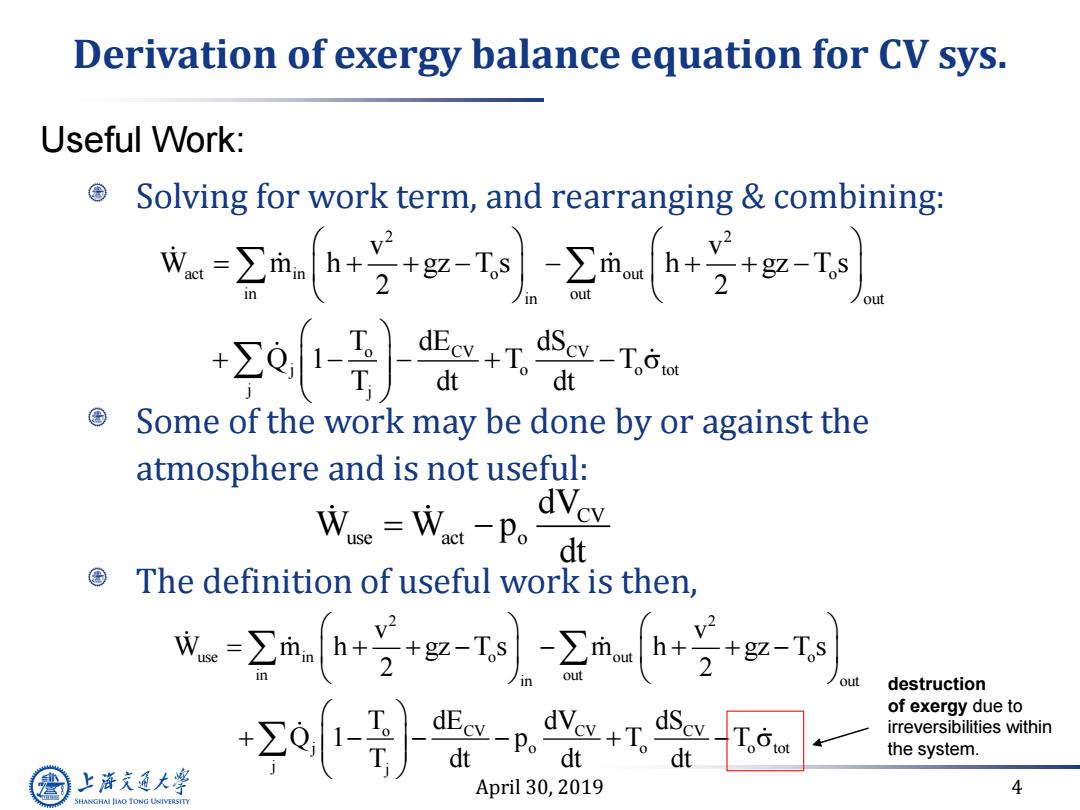
Derivation of exergy balance equation for CV sys. Useful Work: Solving for work term,and rearranging combining: w-m hg-ta mahst) +o-}监+会- dt Some of the work may be done by or against the atmosphere and is not useful: We=Wca-P。 dVev dt The definition of useful work is then, ma++g-T-mn+号g-t out destruction +-} dEcv-Po dScv of exergy due to irreversibilities within dt the system. 上游充通大学 April 30,2019 4 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 4 Derivation of exergy balance equation for CV sys. Solving for work term, and rearranging & combining: Some of the work may be done by or against the atmosphere and is not useful: The definition of useful work is then, 2 2 act in o out o in out in out o CV CV j o o tot j j v v W m h gz T s m h gz T s 2 2 T dE dS Q 1 T T T dt dt CV use act o dV W W p dt Useful Work: 2 2 use in o out o in out in out o CV CV CV j o o o tot j j v v W m h gz T s m h gz T s 2 2 T dE dV dS Q 1 p T T T dt dt dt destruction of exergy due to irreversibilities within the system
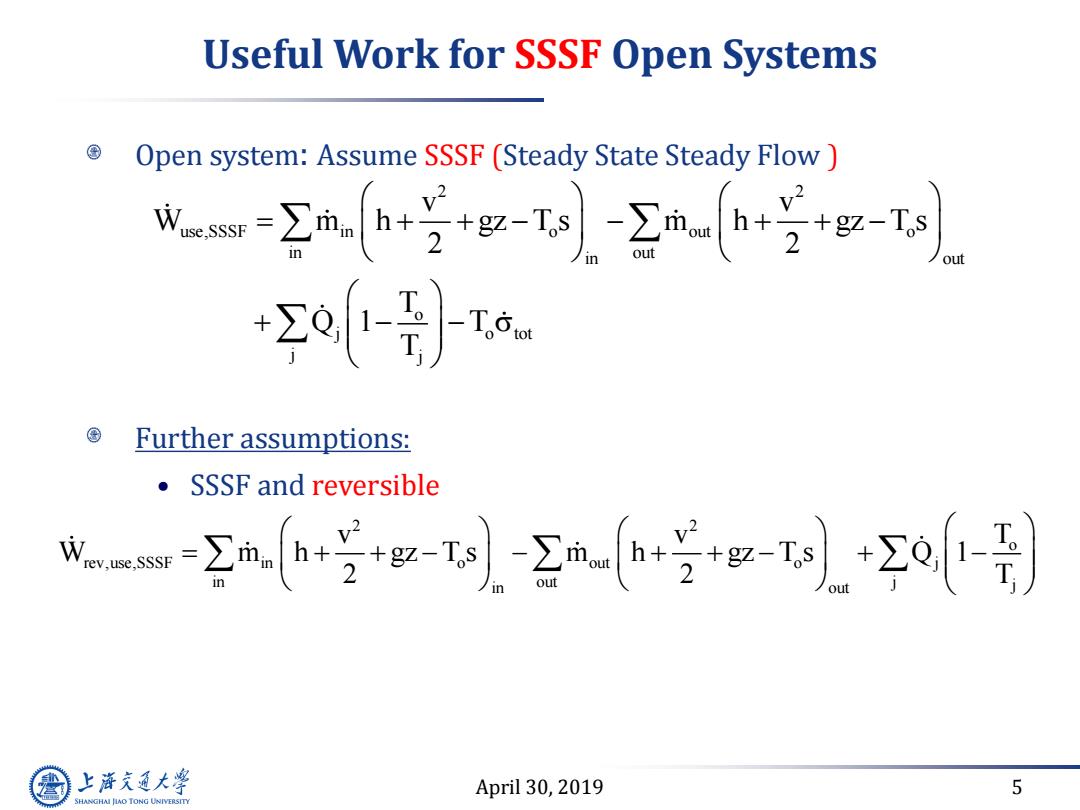
Useful Work for SSSF Open Systems Open system:Assume SSSF (Steady State Steady Flow 成-Σg-T-2h片+g ,v2 o-}- Further assumptions: ●SSSF and reversible wmm-空-g--2+5g以-2o-) 上游通大学 April 30,2019 5 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 5 Useful Work for SSSF Open Systems Open system: Assume SSSF (Steady State Steady Flow ) Further assumptions: • SSSF and reversible 2 2 use,SSSF in o out o in out in out o j o tot j j v v W m h gz T s m h gz T s 2 2 T Q 1 T T 2 2 o rev,use,SSSF in o out o j in out j j in out v v T W m h gz T s m h gz T s Q 1 2 2 T
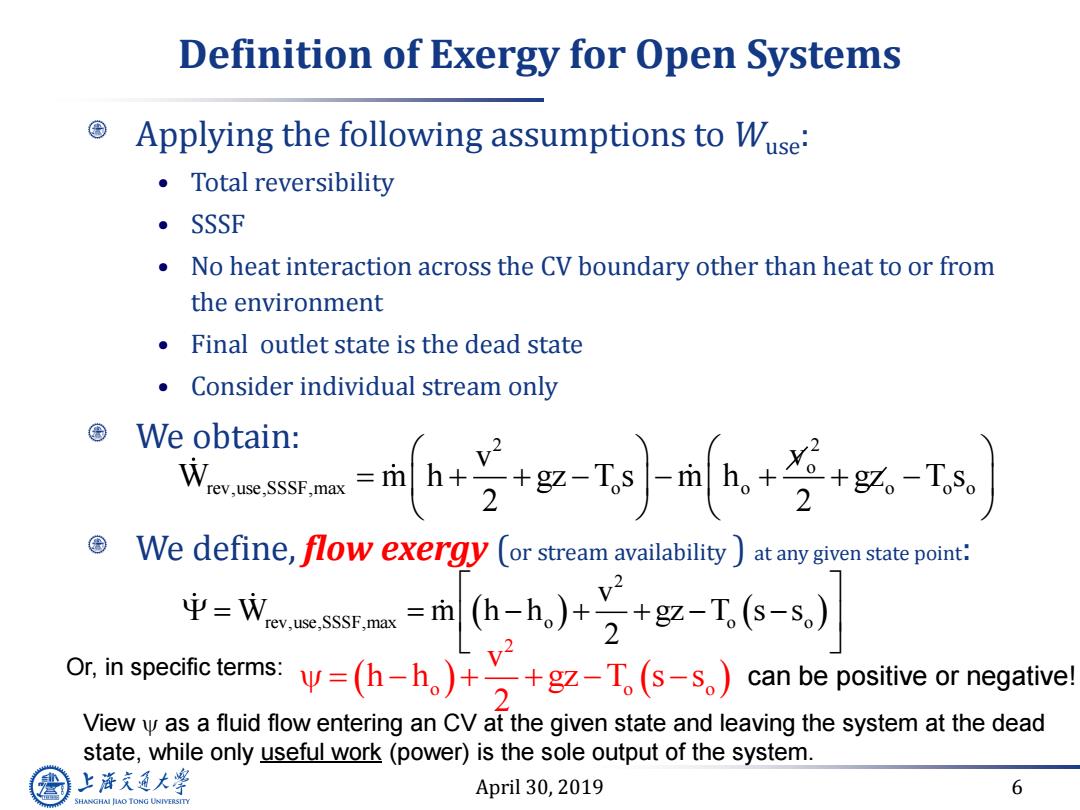
Definition of Exergy for Open Systems Applying the following assumptions to Wuse: ·Total reversibility ·SSSF No heat interaction across the CV boundary other than heat to or from the environment Final outlet state is the dead state Consider individual stream only We obtain: an4g时ja+学+以-T We define,flow exergy (or stream availability)at any given state point: 中=Wwm=mhh,)+2+g-工6-s,) emsy=-h,)十y+gz-T,s-s,)canb View y as a fluid flow entering an CV at the given state and leaving the system at the dead state,while only useful work(power)is the sole output of the system. 上游充通大学 April 30,2019 6 HANGHAI JAO TONG UNIVERSITY
April 30, 2019 6 Definition of Exergy for Open Systems 2 2 o rev,use,SSSF,max o o o o o v v W m h gz T s m h gz T s 2 2 Applying the following assumptions to Wuse: • Total reversibility • SSSF • No heat interaction across the CV boundary other than heat to or from the environment • Final outlet state is the dead state • Consider individual stream only We obtain: We define, flow exergy (or stream availability ) at any given state point: 2 rev,use,SSSF,max o o o v W m h h gz T s s 2 2 o o o v h h gz T s s 2 Or, in specific terms: can be positive or negative! View as a fluid flow entering an CV at the given state and leaving the system at the dead state, while only useful work (power) is the sole output of the system

Change in specific flow exergy for one individual stream across CV inlet and outlet states: △Ψ12=Ψ2-Ψ1 Aye=,-h,)+(-)+g(a-Z)-T(s,-s) Generalizing for multiple inlets and outlets,we define total change in exergy across CV boundary: △中cy=∑loout-∑inVn out in a-Ta out Relationship between flow exergy and useful work for CV: n dt out dc-P。at o dt =0 for SSSF assumption 上游气通大粤 April 30,2019 7 SHANGHAI JLAO TONG UNIVERSITY
April 30, 2019 7 Change in specific flow exergy for one individual stream across CV inlet and outlet states: Generalizing for multiple inlets and outlets, we define total change in exergy across CV boundary: Relationship between flow exergy and useful work for CV: 2 2 12 2 1 2 1 12 2 2 2 1 1 o 1 1 h h v v g z z T s s 2 CV out out in in out in 2 2 CV out o in o out in out in m m v v m h gz T s m h gz T s 2 2 o CV CV CV use in in out out j o tot o o in out j j T dE dV dS W m m Q 1 T p T T dt dt dt 0 for SSSF assumption
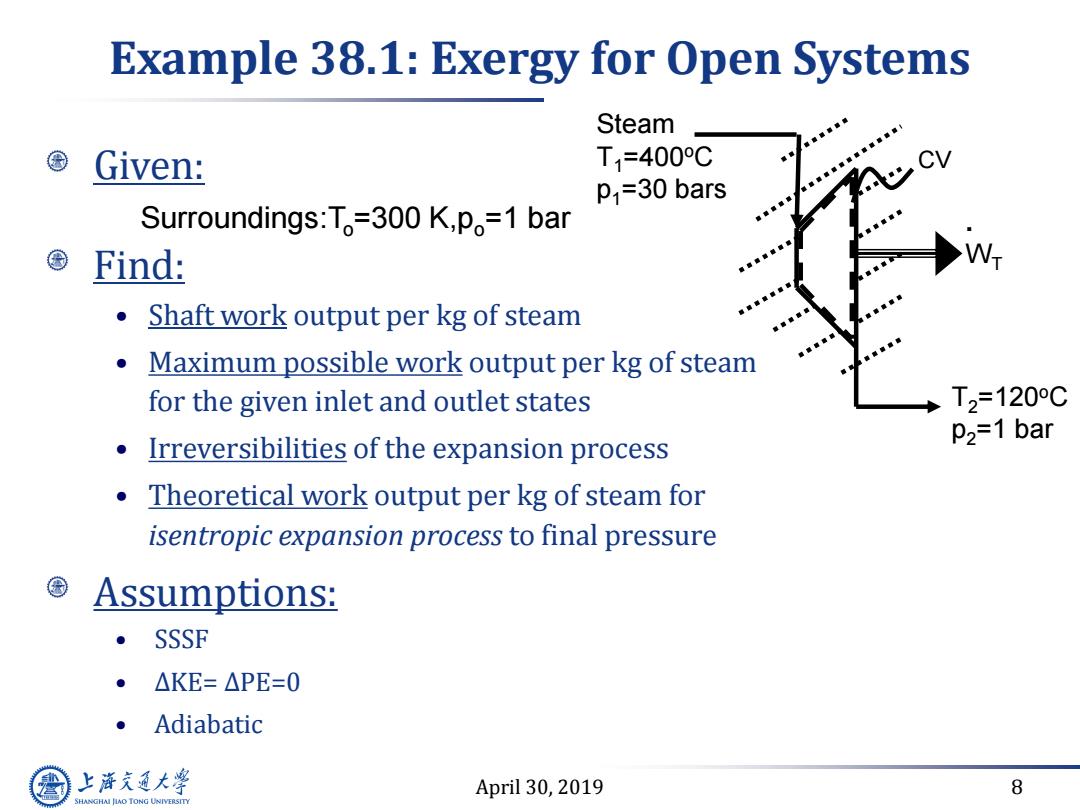
Example 38.1:Exergy for Open Systems Steam Given: T1=400C CV P1=30 bars Surroundings:T=300 K,po=1 bar Find: e Shaft work output per kg of steam Maximum possible work output per kg of steam for the given inlet and outlet states T2=120C P2=1 bar Irreversibilities of the expansion process Theoretical work output per kg of steam for isentropic expansion process to final pressure Assumptions: SSSF ● △KE=△PE=0 。Adiabatic 上游充通大 April 30,2019 8 SHANGHAI JLAO TONG UNIVERSITY
April 30, 2019 8 Example 38.1: Exergy for Open Systems Given: Find: • Shaft work output per kg of steam • Maximum possible work output per kg of steam for the given inlet and outlet states • Irreversibilities of the expansion process • Theoretical work output per kg of steam for isentropic expansion process to final pressure Assumptions: • SSSF • ΔKE= ΔPE=0 • Adiabatic . WT CV Steam T1=400oC p1=30 bars T2=120oC p2=1 bar Surroundings:To=300 K,po=1 bar

Continue Example 38.1 Steam Solution: T1=400C P=30 bars ·From steam tables: h1=3230.9kJ/kg s=6.9212kJ/kg·K) h2=2716.6kJ/kg s2=7.4668kJ/(kg·K) T2=120C p2=1 bar 1st law,energy balance: --++差 dt W=minhin-mouhout Mass balaee:i=m。=i w,=h-h,=(3230.9-2716.6)=5143 g 上游通大学 April 30,2019 9 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 9 Continue Example 38.1 Solution: • From steam tables: • 1 st law, energy balance: CV dE dt 2 in v Q W m h 2 gz 2 out out in v m h 2 gz out in in out in in out out T 1 2 Mass balance : m m m W m h m h kJ kJ w h h 3230.9 2716.6 514.3 kg kg 1 1 2 2 h 3230.9 kJ kg s 6.9212 kJ (kg K) h 2716.6 kJ kg s 7.4668 kJ (kg K) . WT CV Steam T1=400oC p1=30 bars T2=120oC p2=1 bar
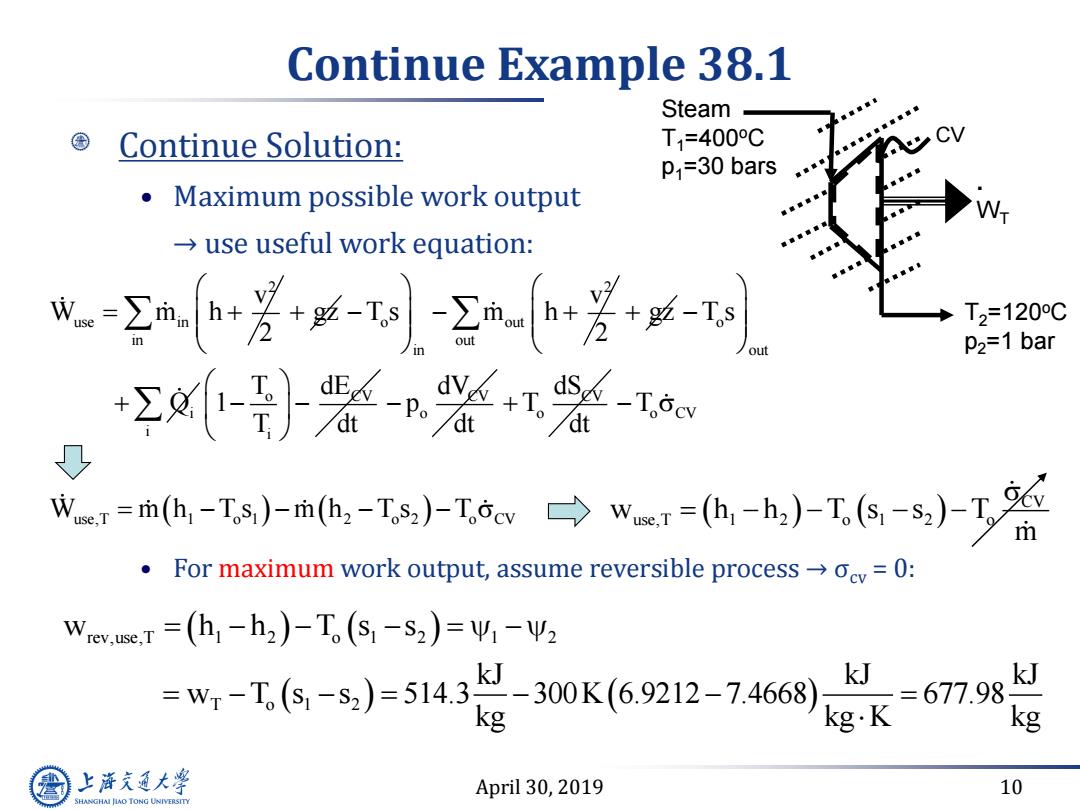
Continue Example 38.1 Steam Continue Solution: T=400C P=30 bars Maximum possible work output use useful work equation: 成+芳--h+- T2=120C P2=1 bar +Σ-}装n兴 dSg-Tocv w1=h-Is)-mh,-Ts,)-Iw→wer=(,-h)-T6-s,)T m For maximum work output,assume reversible processocv=0: wev,c,T=(h-h2)-T(S-S2)=41-Ψ2 =w,-T,6-5,)=5143300K(6.9212-7468) kJ =677.9 kJ kg g.K kg 上游充通大 April 30,2019 10 SHANGHAI JIAO TONG UNIVERSITY
April 30, 2019 10 Continue Example 38.1 Continue Solution: • Maximum possible work output → use useful work equation: • For maximum work output, assume reversible process → σcv = 0: 2 use in v W m h 2 gz 2 o out in in v T s m h 2 gz o out out i T s Q o CV i i T dE 1 T dt CV o dV p dt CV o dS T dt o CV use,T 1 o 1 2 o 2 o CV T W m h T s m h T s T . WT CV Steam T1=400oC p1=30 bars T2=120oC p2=1 bar CV w h h T s s T use,T 1 2 o 1 2 o m rev,use,T 1 2 o 1 2 1 2 T o 1 2 w h h T s s kJ kJ kJ w T s s 514.3 300K 6.9212 7.4668 677.98 kg kg K kg