上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 34 Chapter 7 Entropy (Section 7.13) Spring,4/23/2019 强 Prof.,Dr.Yonghua HUANG Rnn是。 http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 34 Spring, 4/23/2019 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.13) http://cc.sjtu.edu.cn/G2S/site/thermo.html
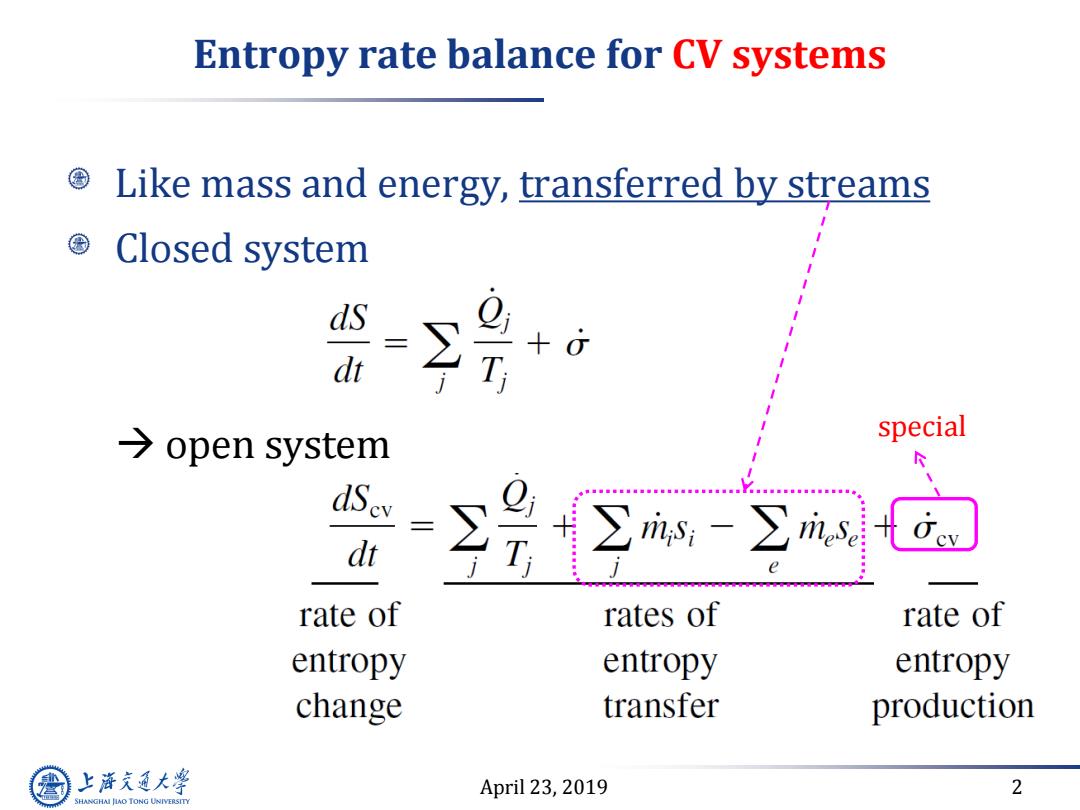
Entropy rate balance for CV systems Like mass and energy,transferred by streams Closed system +o T →open system special dSev = dt ∑m空m rate of rates of rate of entropy entropy entropy change transfer production 上游气通大粤 April 23,2019 2 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 2 Entropy rate balance for CV systems Like mass and energy, transferred by streams Closed system open system special
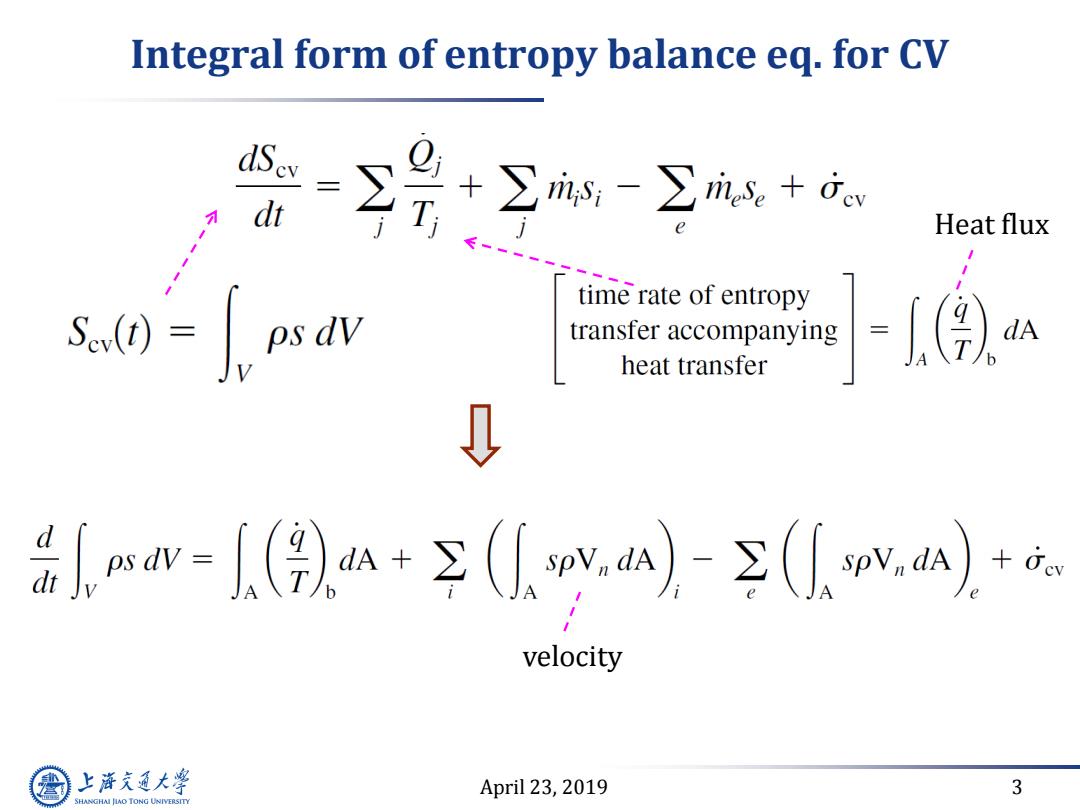
Integral form of entropy balance eq.for CV 号空包 Heat flux time rate of entropy Sev(t) transfer accompanying heat transfer 0 w-)+p)-sp1元 velocity 上游充通大 April 23,2019 3 SHANGHAI JIAO TONG UNIVERSITY
April 23, 2019 3 Integral form of entropy balance eq. for CV Heat flux velocity

CV at steady state Steady state: Mass balance: ∑m=∑m Energy balance: 0-a.-成,+区a(a+是+-Σe+号+s Entropy balance: 70 dS= "'dt 汽区 Not conserved!! These equations often must be solved simultaneously. irreversibilities 上游充通大 April 23,2019 4 SHANGHAI JIAO TONG UNIVERSITY
April 23, 2019 4 CV at steady state Steady state: Mass balance: Energy balance: Entropy balance: 0 These equations often must be solved simultaneously. Not conserved!! irreversibilities
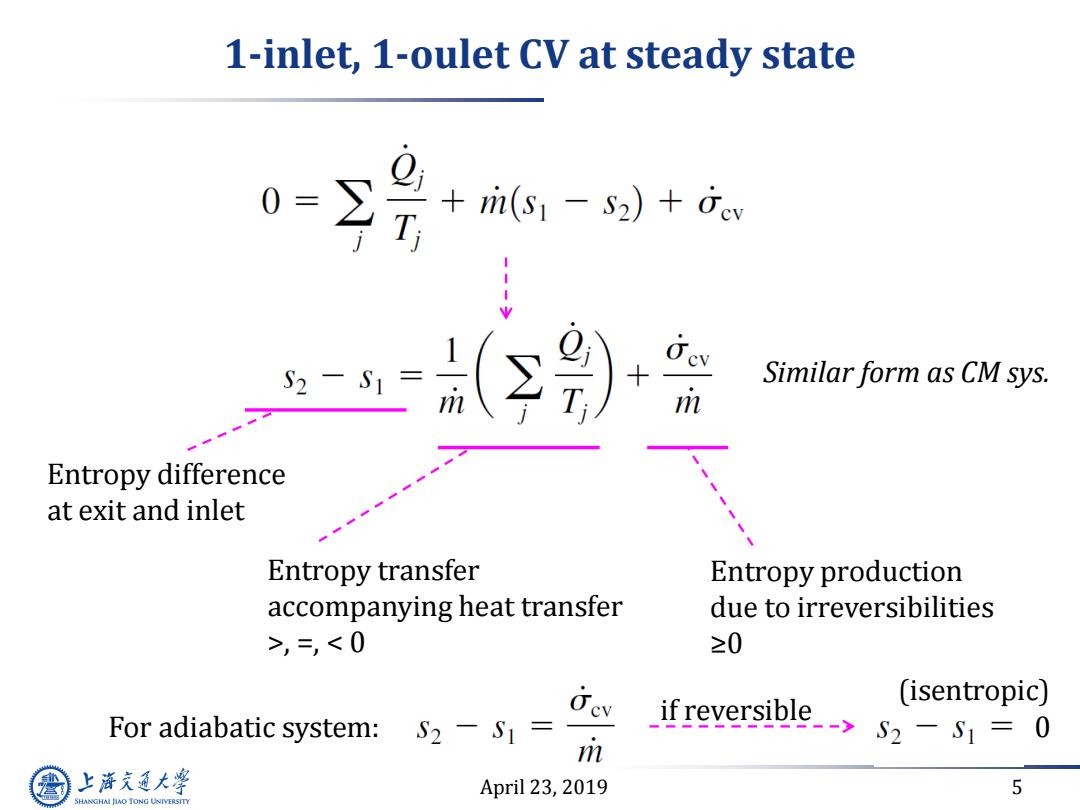
1-inlet,1-oulet CV at steady state 0= T ri(s1 -s2)+fev +r Similar form as CM sys. m Entropy difference at exit and inlet Entropy transfer Entropy production accompanying heat transfer due to irreversibilities >,=,52-S=0 m 上游充通大 April 23,2019 5 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 5 1-inlet, 1-oulet CV at steady state Entropy transfer accompanying heat transfer >, =, < 0 Entropy production due to irreversibilities ≥0 Similar form as CM sys. For adiabatic system: if reversible 0 (isentropic) Entropy difference at exit and inlet

Example 34.1 Entropy Production in a Steam Turbine Known: P1 =30 bar T =400°C Wey =540 kJ/kg steam expands through V1=160m/s a turbine at steady state. steam Find: Gcv/m D>T2=100C Tb=350K- Saturated vapor V2=100m/s Sketch 30 bar 400°C Assumption: 1.CV,(1,1,1)steady state 2.Heat transfer at T 3.△PE neglected 100C 上游充通大学 April 23,2019 6 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 6 Example 34.1 Entropy Production in a Steam Turbine Find: steam Known: steam expands through a turbine at steady state. CV / m Assumption: 1. CV, (1,1,1) steady state 2. Heat transfer at Tb 3. ∆PE neglected Sketch ?
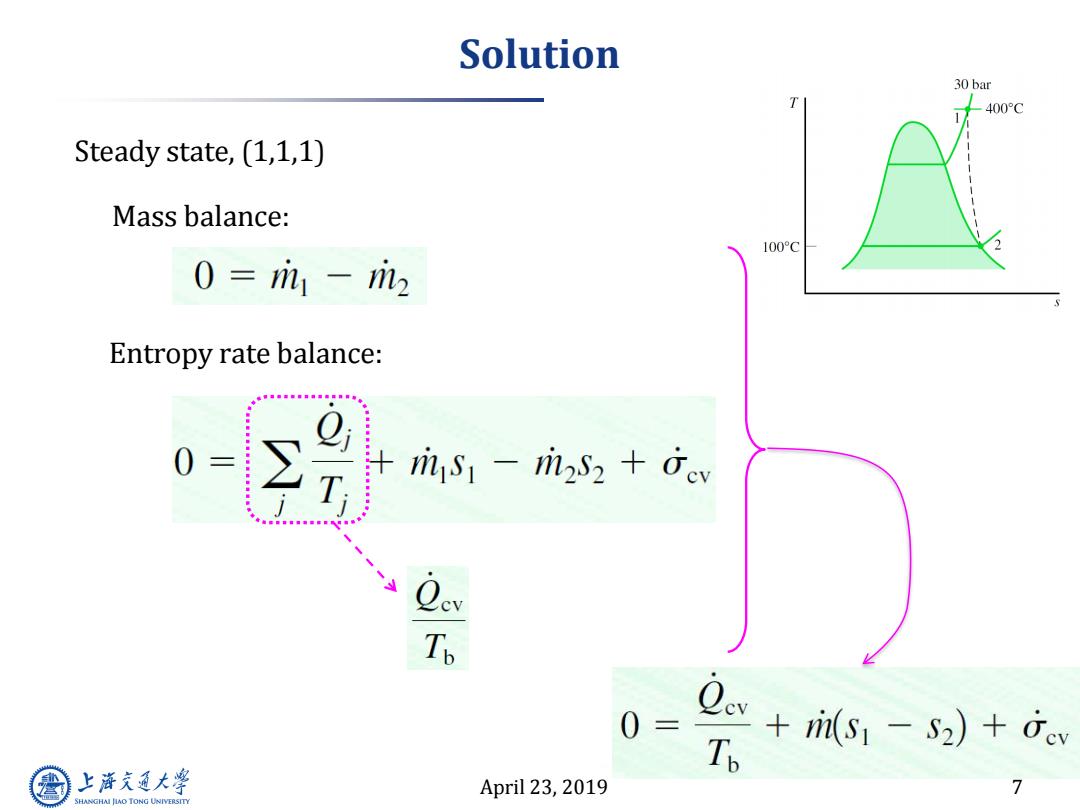
Solution 30 bar 400°C Steady state,(1,1,1) Mass balance: 100C 0=m1-2 Entropy rate balance: ris-mi2s2 +ev To 0 三 s+is)o T 上游究通大学 April 23,2019 7 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 7 Solution Steady state, (1,1,1) Mass balance: Entropy rate balance:

Solution cont. 30 bar 400°C Tp +(S1-S2)+0cv 100°C Q./m 个 (S2-S1) m Energy balance equation:∠? -++(明 i TabA-6,(30bar,400C→h1=3230.9k/kg; TabA-4,(100C)→h2=hg=2676.1k/kg -sn是+eos1--[7,@1(g)hlml 1kJ m kg =540-554.8-7.8=-22.6kJ/kg 上游充通大学 April 23,2019 8 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 8 Solution cont. Energy balance equation: ?? Tab A-6, (30bar, 400˚C)h1 = 3230.9 kJ/kg; Tab A-4, (100˚C)h2=hg = 2676.1 kJ/kg ??

Solution cont. 30bar 400°C TabA-4,(100C)→s2=7.3549k/kg TabA-6,(30bar,400°C)→s1=6.9212k/kg-K; 100°C -22.6kJ/kg、 Qev/ri +(S2-S1) m Tp (-22.6kJkg) 350K +(7.3549-6.9212) = 0.0646+0.4337=0.4983kJ/kg·K 上游充通大 April 23,2019 9 SHANGHAI JIAO TONG UNIVERSITY
April 23, 2019 9 Solution cont. Tab A-6, (30bar, 400˚C)s1 = 6.9212kJ/kg-K; Tab A-4, (100˚C)s2=7.3549 kJ/kg
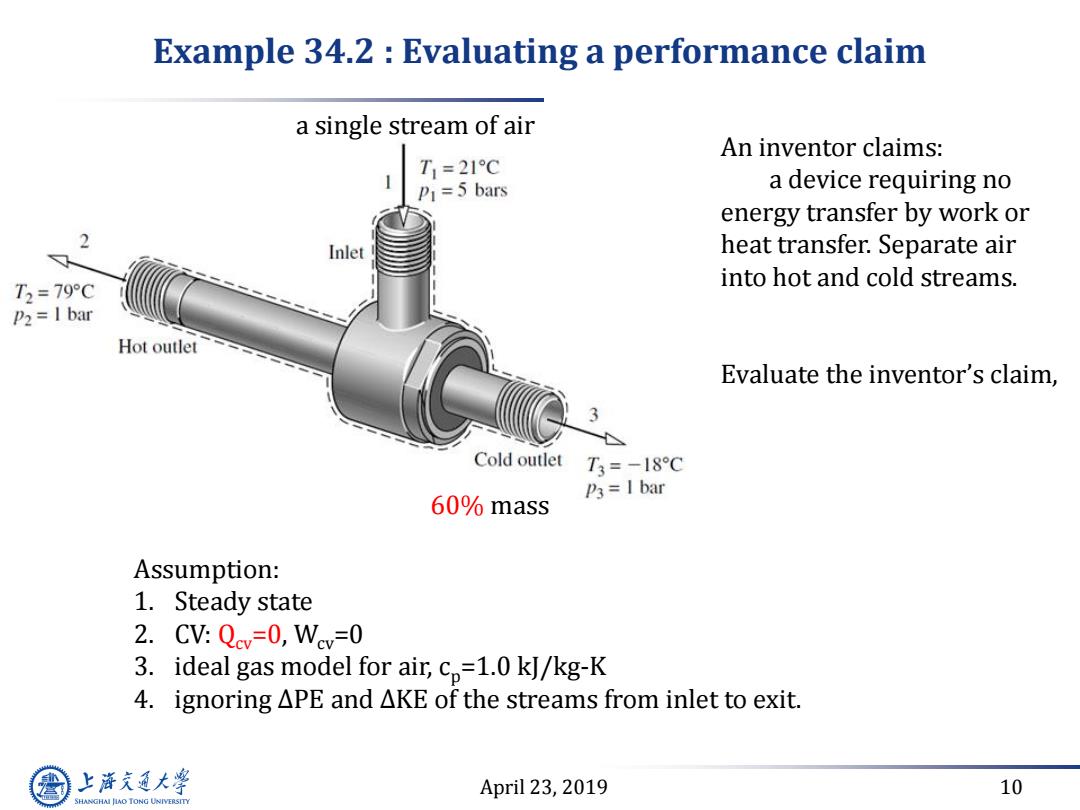
Example 34.2 Evaluating a performance claim a single stream of air An inventor claims: T1=21C P1=5 bars a device requiring no energy transfer by work or 2 Inlet heat transfer.Separate air T2=79C into hot and cold streams. P2=I bar Hot outlet Evaluate the inventor's claim, Cold outlet T3=-18C P3=I bar 60%mass Assumption: 1.Steady state 2.CV:Qcv=0,Wcv=0 3. ideal gas model for air,cp=1.0 kJ/kg-K 4.ignoring APE and AKE of the streams from inlet to exit. 上游充通大 April 23,2019 10 SHANGHAI JLAO TONG UNIVERSITY
April 23, 2019 10 Example 34.2 : Evaluating a performance claim An inventor claims: a device requiring no energy transfer by work or heat transfer. Separate air into hot and cold streams. Evaluate the inventor’s claim, a single stream of air 60% mass Assumption: 1. Steady state 2. CV: Qcv=0, Wcv=0 3. ideal gas model for air, cp=1.0 kJ/kg-K 4. ignoring ∆PE and ∆KE of the streams from inlet to exit