
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 35 Chapter 7 Entropy Section7.4,7.9,7.12) Spring,4/27/2018 Prof.,Dr.Yonghua HUANG 强 L是。 http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 35 Spring, 4/27/2018 Prof., Dr. Yonghua HUANG Chapter 7 Entropy (Section 7.4, 7.9, 7.12) http://cc.sjtu.edu.cn/G2S/site/thermo.html
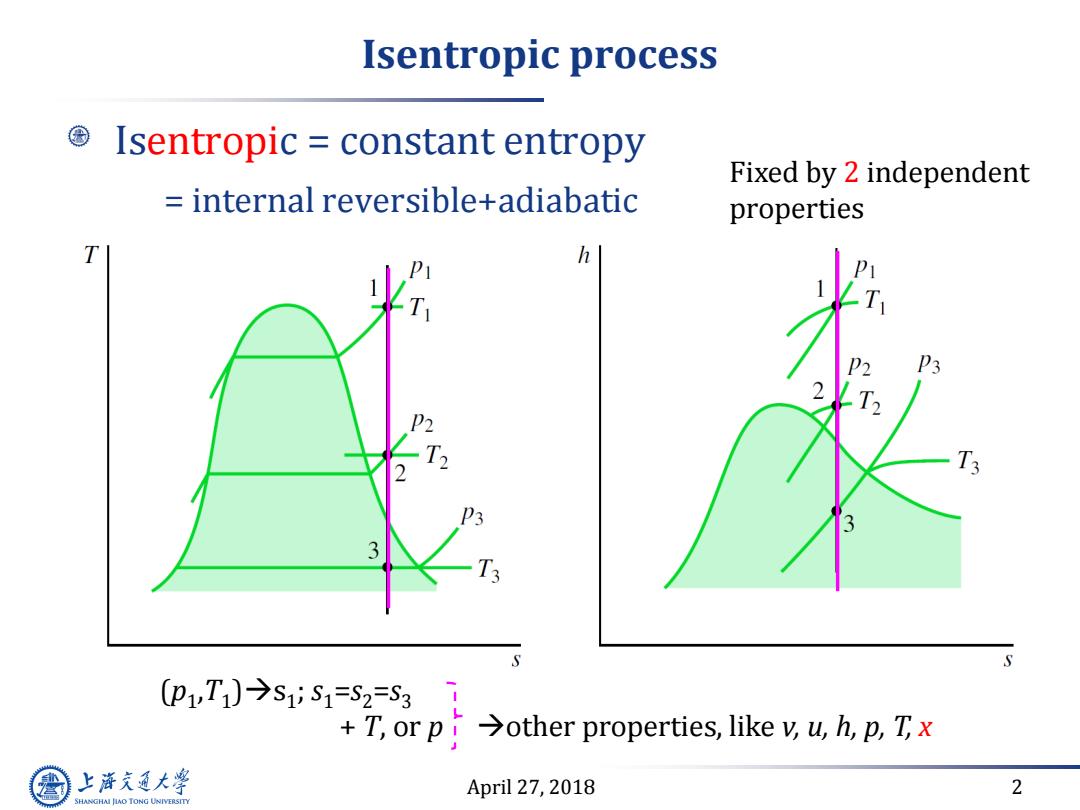
Isentropic process Isentropic constant entropy Fixed by 2 independent internal reversible+adiabatic properties T h P3 2 T3 p1,T1)今1iS1=S2=S31 T,or p>other properties,like v,u,h,p,Tx 上游充通大 April 27,2018 2 SHANGHAI JLAO TONG UNIVERSITY
April 27, 2018 2 Isentropic process Isentropic = constant entropy = internal reversible+adiabatic Fixed by 2 independent properties (p1 ,T1 )s1 ; s1=s2=s3 + T, or p other properties, like v, u, h, p, T, x
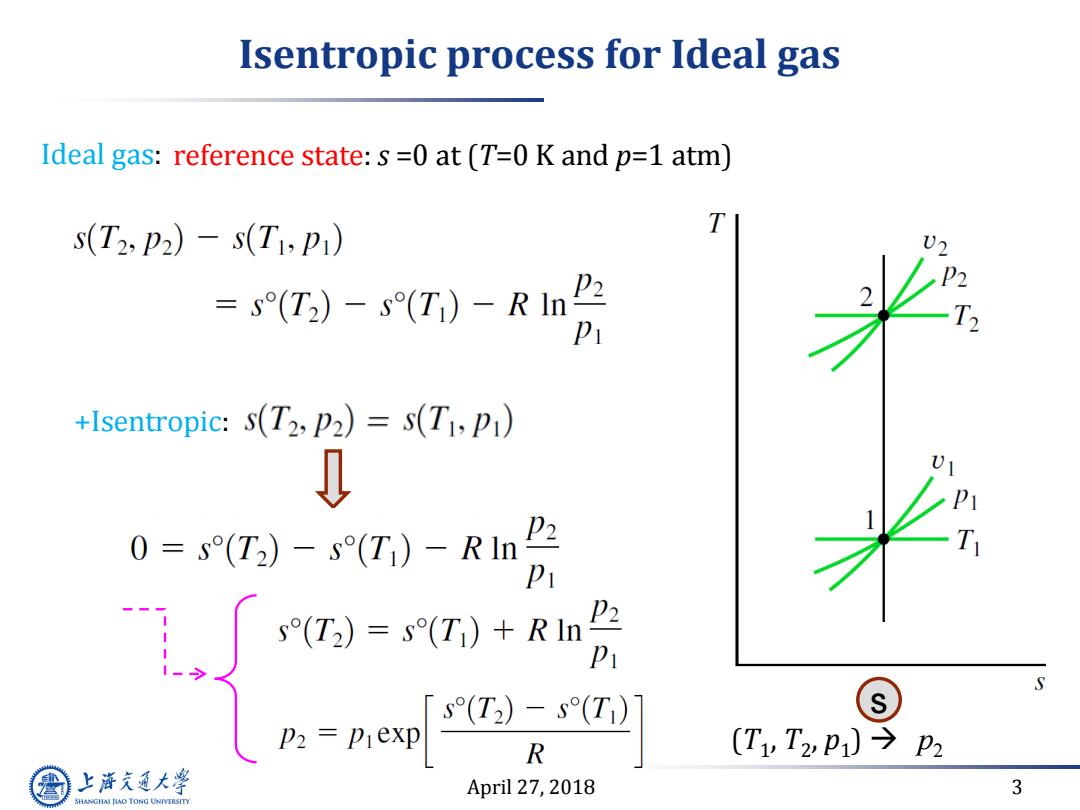
Isentropic process for Ideal gas Ideal gas:reference state:s=0 at(T=0 K and p=1 atm) s(T2:P2)-s(Ti,P) =s(T2)-s(T)-RIn2 P2 +Isentropic:s(T2,P2)=s(T1,p) 0 P2 0=s(T2)-s(T)-RIn =g) s(T2)=s(T)+RIn P2 Pi S R (T1,T2z,p1)今P2 上游通大学 April 27,2018 3 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 3 Isentropic process for Ideal gas Ideal gas: reference state: s =0 at (T=0 K and p=1 atm) +Isentropic: (T1 , T2 , p1 ) p2 s
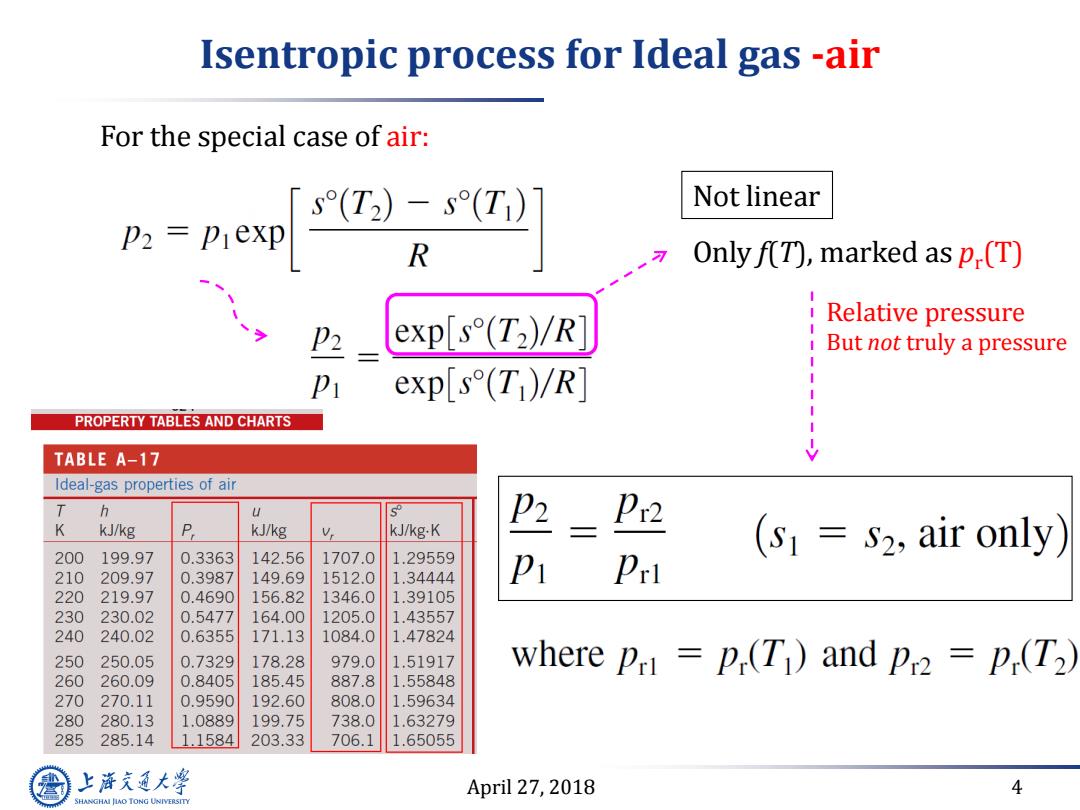
Isentropic process for Ideal gas -air For the special case of air: D2 prexp 「s(T2)-s(T) Not linear Only f(T),marked as p.(T) exp[s(Tz)/R] Relative pressure P2 But not truly a pressure exp[s(T)/R PROPERTY TABLES AND CHARTS TABLE A-17 Ideal-gas properties of air T h U Pr2 K kJ/kg kJ/kg Vr kJ/kg.K (s S2,air only) 200 199.97 0.3363 142.56 1707.0 1.29559 210 209.97 0.3987 149.69 1512.0 1.34444 Pr 220 219.97 0.4690 156.82 1346.0 1.39105 230 230.02 0.5477 164.00 1205.0 1.43557 240 240.02 0.6355 171.13 1084.0 1.47824 250 250.05 0.7329 178.28 979.0 1.51917 where pri p(T1)and pr2 p(T2) 260 260.09 0.8405 185.45 887.8 1.55848 270 270.11 0.9590 192.60 808.0 1.59634 280 280.13 1.0889 199.75 738.0 1.63279 285 285.14 1.1584 203.33 706.1 1.65055 上泽通大学 April 27,2018 4 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 4 Isentropic process for Ideal gas -air Only f(T), marked as pr (T) For the special case of air: Relative pressure But not truly a pressure Not linear

Isentropic process for Ideal gas -air,cont. For the special case of air: -(网a)… P2 Pr (s=S2,air only Only f(T),marked as v(T) Pr Relative volume PROPERTY TABLES AND CHARTS But not truly a volume TABLE A-17 Ideal-gas properties of air U2 T t s U2 K kJ/kg P kJ/kg V. kJ/kg.K (s=52,air only) 200199.97 0.3363 142.56 1707.0 1.29559 Url 210 209.97 0.3987 149.69 1512.0 1.34444 220 219.97 0.4690 156.82 1346.0 1.39105 230 230.02 0.5477 164.00 1205.0 1.43557 240 240.02 0.6355 171.13 1084.0 1.47824 250 250.05 0.7329 178.28 979.0 1.51917 260 260.09 0.8405 185.45 887.8 1.55848 where v =v(T1)and v2=v(T2) 270 270.11 0.9590 192.60 808.0 1.59634 280 280.13 1.0889 199.75 738.0 1,63279 285 285.14 11584 203.33 706.1 1.65055 上通大学 April 27,2018 5 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 5 Isentropic process for Ideal gas –air, cont. For the special case of air: Only f(T), marked as vr (T) Relative volume But not truly a volume where vr1 = vr (T1 ) and vr2 = vr (T2 )
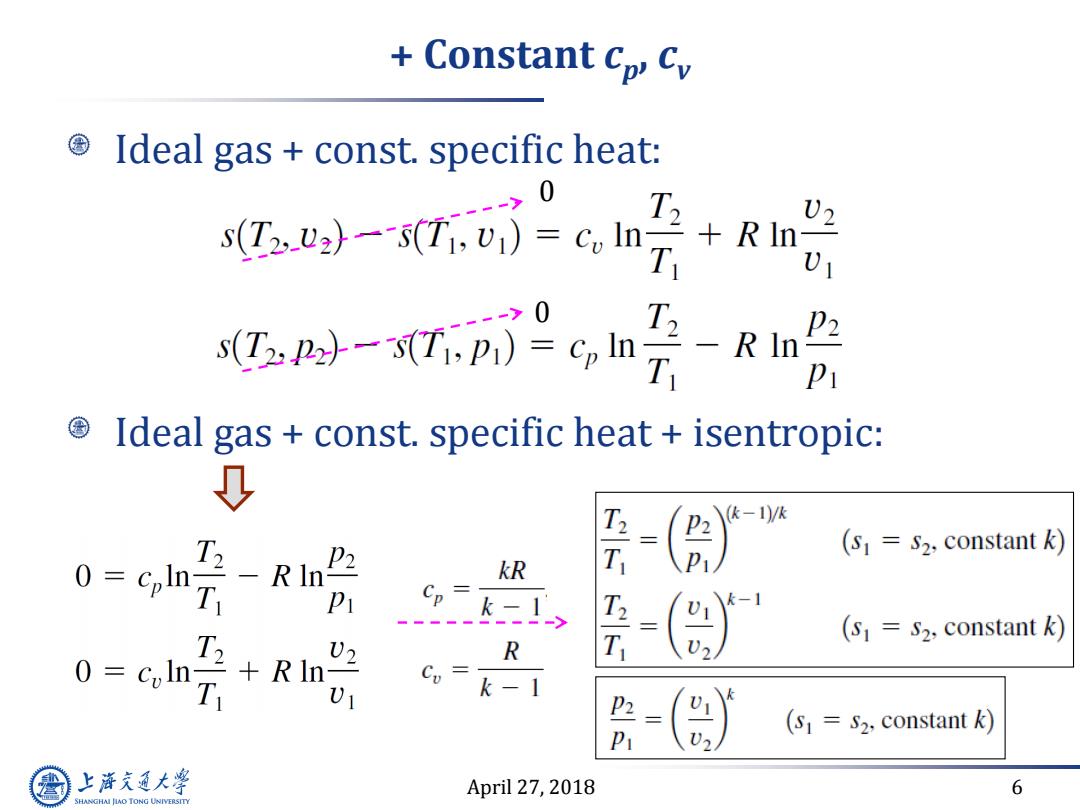
Constant cp Cv Ideal gas const.specific heat: 0 s(T2 U2)--s(Ti,v1)=coIn+R In T V ->0 T2 sP-Tp)=Gl P2 pi Ideal gas const.specific heat isentropic: 0 (k-1)/k T2 P2 巧刀 (s=S2,constant k) 0= kR Pi Cp= - k-I T2 T (s1=S2,constant k) R 0=colr 02 +R In- D T Ui c=k-1 (S1 S2,constant k) P 上游气通大粤 April 27,2018 6 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 6 + Constant cp , cv Ideal gas + const. specific heat: Ideal gas + const. specific heat + isentropic: 0 0
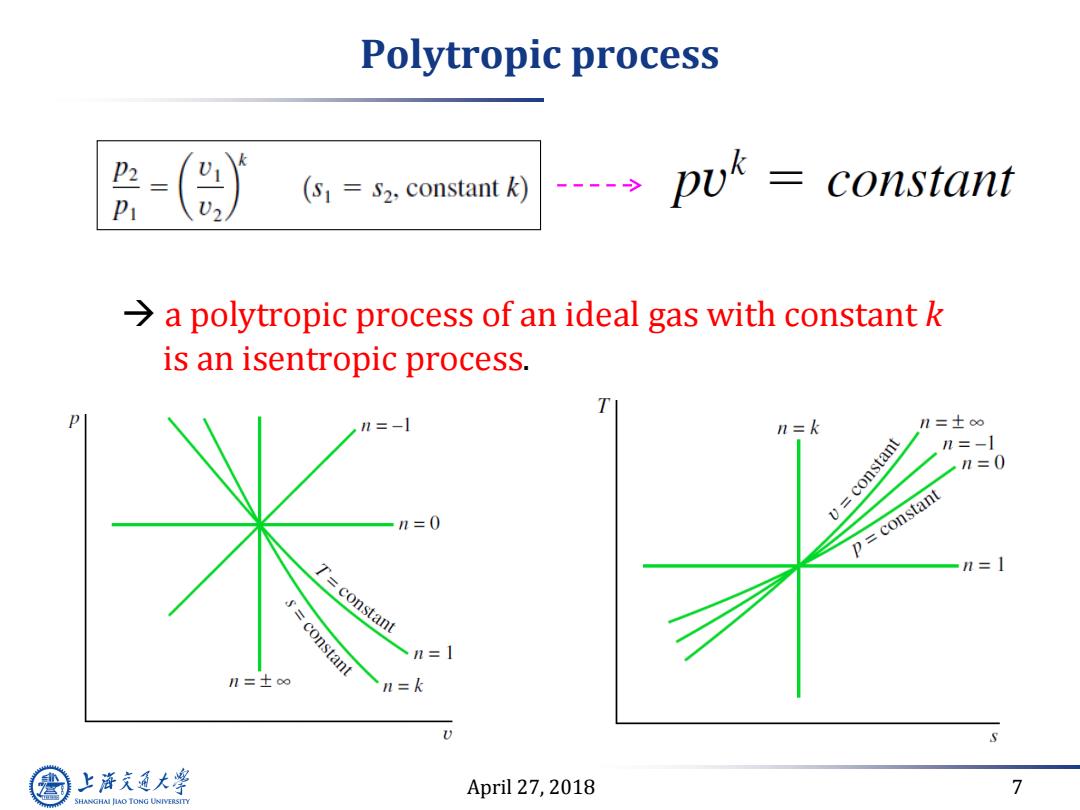
Polytropic process (S1=S2.constant k) 。pwk= constant a polytropic process of an ideal gas with constant k is an isentropic process. P n=-1 n =k n=±oo v=constant n=-1 n=0 n=0 p constant n=1 T=constant constant n=1 n=士o n=k S 上游充通大 April 27,2018 7 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 7 Polytropic process a polytropic process of an ideal gas with constant k is an isentropic process
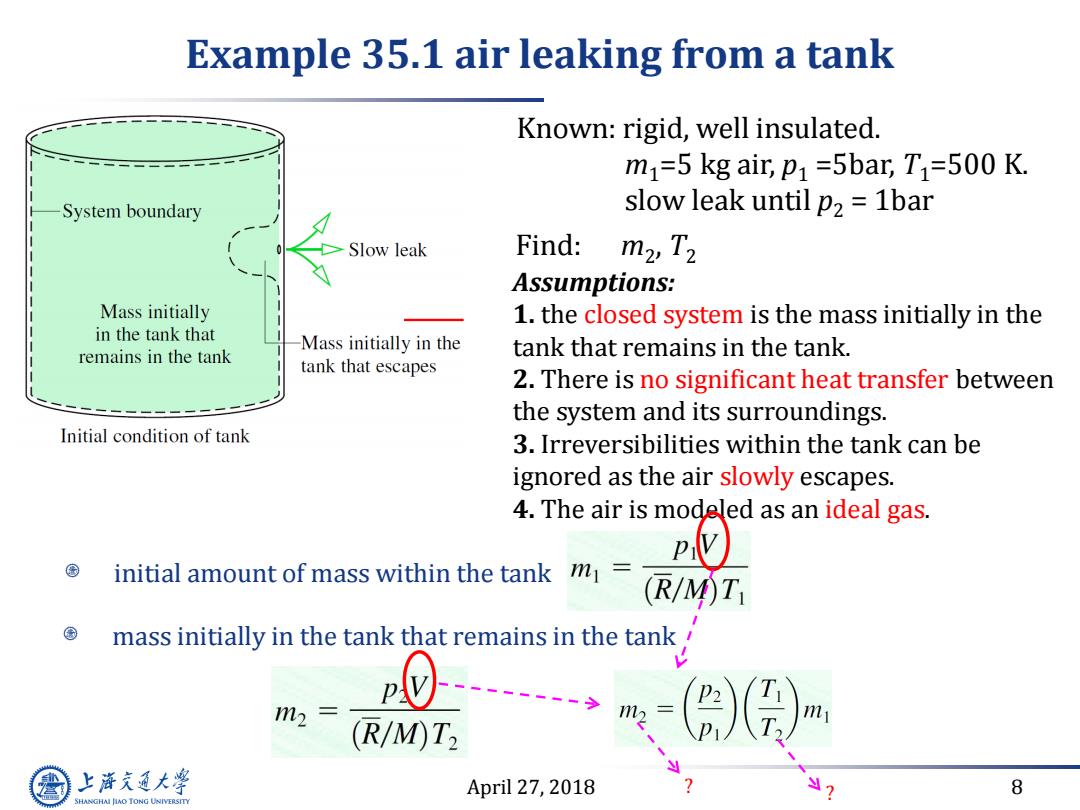
Example 35.1 air leaking from a tank Known:rigid,well insulated. m1=5 kg air,p1 =5bar,T1=500 K. System boundary slow leak until p2 1bar Slow leak Find: m2,T2 Assumptions: Mass initially 1.the closed system is the mass initially in the in the tank that Mass initially in the remains in the tank tank that remains in the tank. tank that escapes 2.There is no significant heat transfer between the system and its surroundings. Initial condition of tank 3.Irreversibilities within the tank can be ignored as the air slowly escapes. 4.The air is modeled as an ideal gas. initial amount of mass within the tank m= (R/M)T mass initially in the tank that remains in the tank 1m2 (R/M)T2 上游充通大 April 27,2018 8 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 8 Example 35.1 air leaking from a tank Known: rigid, well insulated. m1=5 kg air, p1 =5bar, T1=500 K. slow leak until p2 = 1bar Find: m2 , T2 Assumptions: 1. the closed system is the mass initially in the tank that remains in the tank. 2. There is no significant heat transfer between the system and its surroundings. 3. Irreversibilities within the tank can be ignored as the air slowly escapes. 4. The air is modeled as an ideal gas. mass initially in the tank that remains in the tank initial amount of mass within the tank ? ?
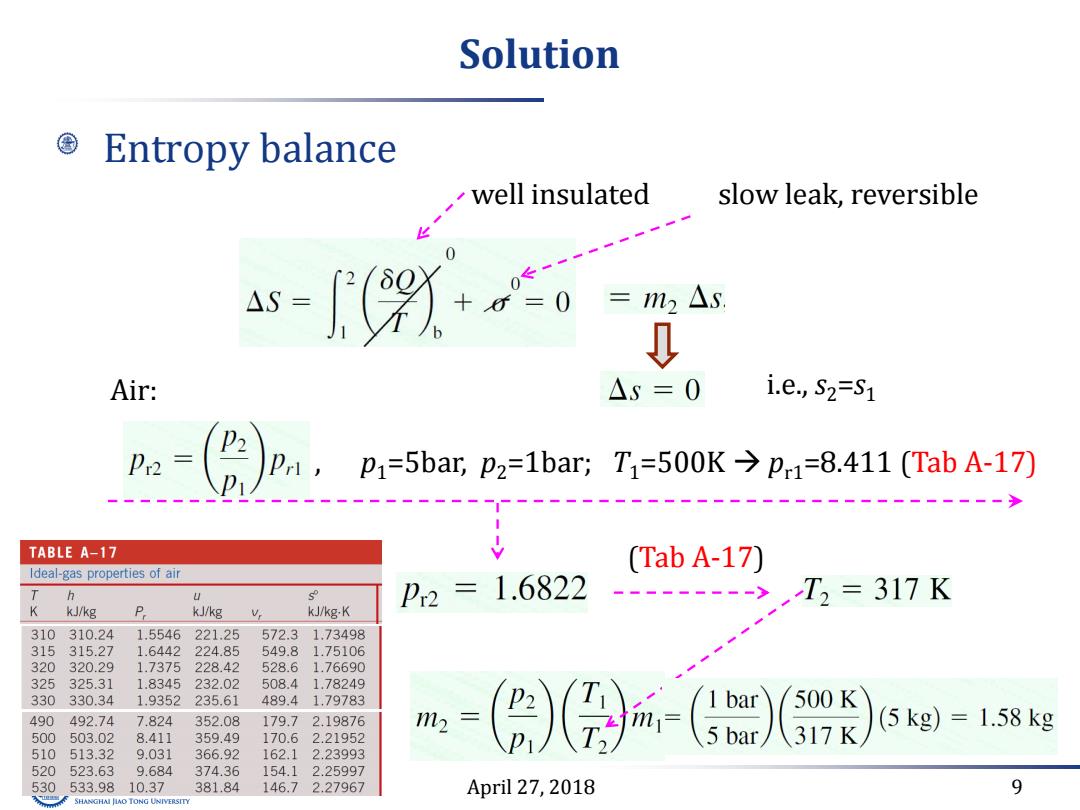
Solution Entropy balance well insulated slow leak,reversible a-+0 0 =m2△s Air: △S=0 i.e.,S2=S1 P2 P2= Pri,p1=5bar,p2=1bar; T1=500K→p1=8.411(TabA-17) TABLE A-17 (Tab A-17) Ideal-gas properties of air T s P2=1.6822 ------>,T2=317K K kJ/kg P kJ/kg kJ/kg-K 310310.24 1.5546 221.25 572.3 1.73498 315315.27 1.6442 224.85 549.8 1.75106 320 320.29 1.7375 228.42 528.6 1.76690 325 325.31 1.8345 232.02 508.4 1.78249 330330.34 1.9352235.61 489.4 1.79783 m2 500K 490 492.74 7.824 352.08 179.7 2.19876 317K (5kg)=1.58kg 500 503.02 8.411 359.49 170.62.21952 510513.32 9.031 366.92 162.1 2.23993 520 523.63 9.684 374.36 154.12.25997 530 533.9810.37 381.84 146.72.27967 April 27,2018 9 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 9 Solution Entropy balance well insulated slow leak, reversible i.e., s2=s Air: 1 , p1=5bar, p2=1bar; T1=500K pr1=8.411 (Tab A-17) (Tab A-17)

Isentropic efficiency Actual performance of a device Idealized device for the same inlet comparison state and same exit pressure Turbine:(expansion) Energy balance -T Actual expansion m (actual) h1-h2 h-h2s Isentropic expansion Minimum h2? 2 2s Constrain: 个 Accessible Oev states m =S2-S1≥0 P2 "="Adiabatic,no internal irreversibility 上游充通大学 April 27,2018 10 SHANGHAI JIAO TONG UNIVERSITY
April 27, 2018 10 Isentropic efficiency Actual performance of a device Idealized device for the same inlet state and same exit pressure comparison Turbine: (expansion) Energy balance Minimum h2 ? Constrain: “=”: Adiabatic, + no internal irreversibility (actual)