上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 29 Chapter 6 The second law of Thermodynamics (Section 6.7-6.11) Spring,4/18/2018 强 Prof.,Dr.Yonghua HUANG MMMMAMMAA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 29 Spring, 4/18/2018 Prof., Dr. Yonghua HUANG Chapter 6 The second law of Thermodynamics (Section 6.7- 6.11) http://cc.sjtu.edu.cn/G2S/site/thermo.html
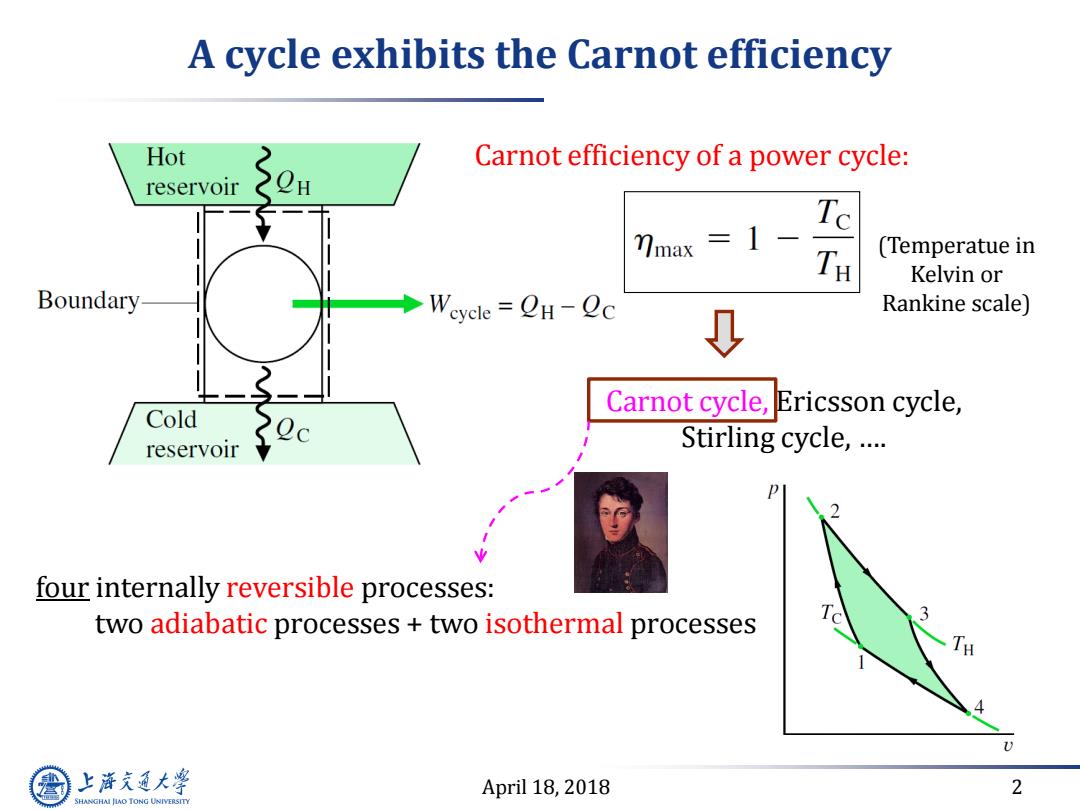
A cycle exhibits the Carnot efficiency Hot Carnot efficiency of a power cycle: reservoir H Te mmax 1- (Temperatue in Kelvin or Boundary →Wevcle=QH-Qc Rankine scale) Cold Carnot cycle,Ericsson cycle, reservoir Stirling cycle,… four internally reversible processes: two adiabatic processes two isothermal processes 3 _TH C 上游充通大 April 18,2018 2 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 2 A cycle exhibits the Carnot efficiency Carnot cycle, Ericsson cycle, Stirling cycle, …. Carnot efficiency of a power cycle: four internally reversible processes: two adiabatic processes + two isothermal processes (Temperatue in Kelvin or Rankine scale)
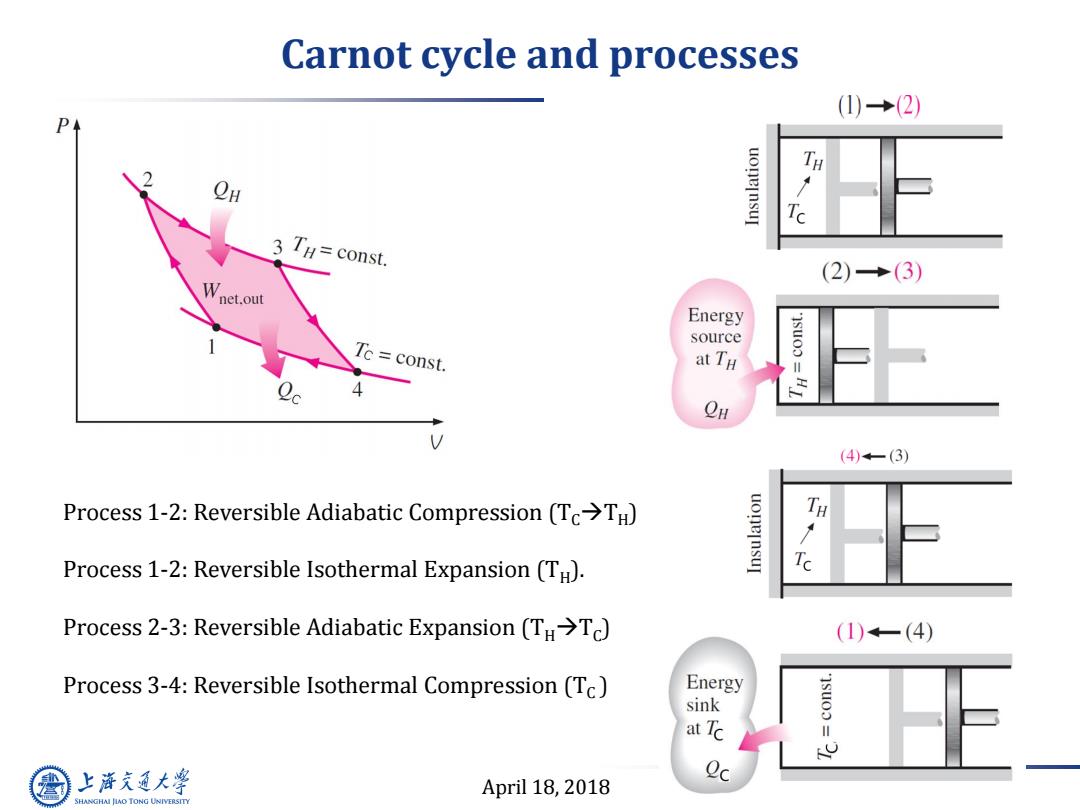
Carnot cycle and processes (1)→(2) uone]nsu Ta 3 TH=const. (2)→(3) Wnet.out Energy source Tc const. at TH 4 (4)一(3) Process 1-2:Reversible Adiabatic Compression (Tc>TH) uonelnsu Ta Process 1-2:Reversible Isothermal Expansion(TH). Process 2-3:Reversible Adiabatic Expansion(TH >Tc) (1)←一(4) Process 3-4:Reversible Isothermal Compression(Tc) Energy sink at TC i 四 上游充通大 April 18,2018 Qc SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 3 Carnot cycle and processes Process 1-2: Reversible Adiabatic Compression (TCTH ) Process 1-2: Reversible Isothermal Expansion (TH ). Process 2-3: Reversible Adiabatic Expansion (THTC ) Process 3-4: Reversible Isothermal Compression (TC )
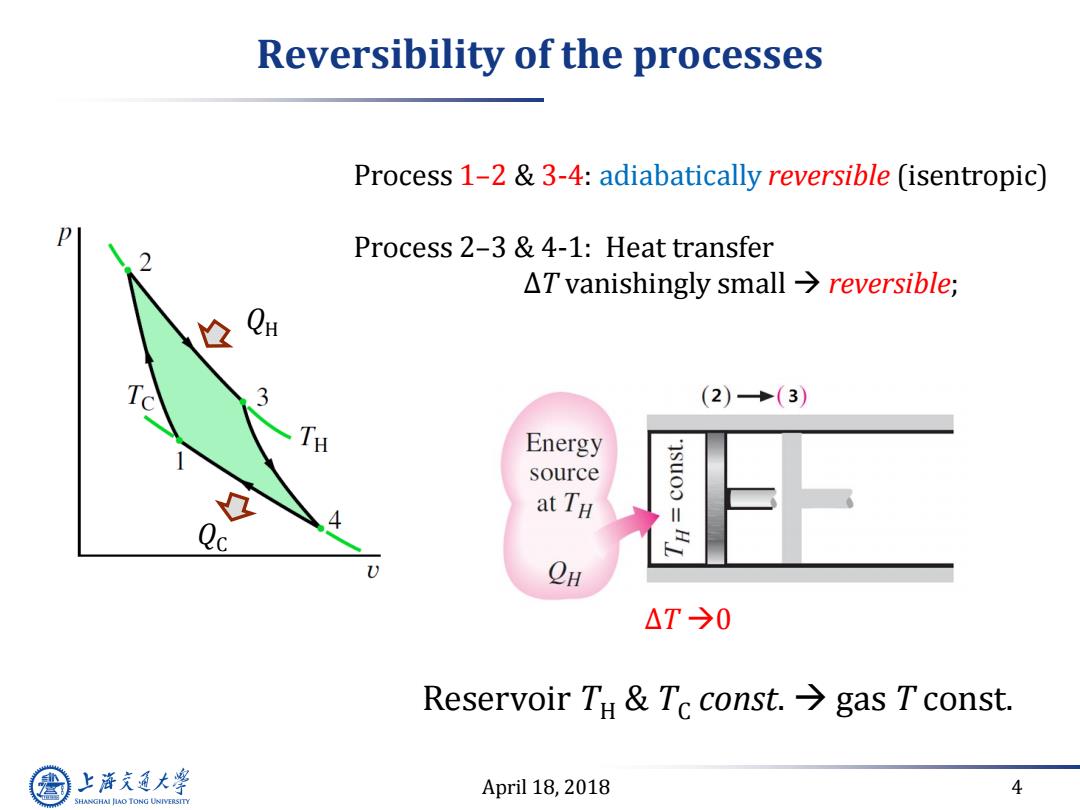
Reversibility of the processes Process 1-2&3-4:adiabatically reversible (isentropic) Process 2-3 &4-1:Heat transfer △I vanishingly small→reversible; 个 (2)·(3) TH Energy source at TH B10 OH △T→0 Reservoir TH Tc const.gas Tconst. 上游充通大学 April 18,2018 4 SHANGHAI JLAO TONG UNIVERSITY
April 18, 2018 4 Reversibility of the processes Process 1–2 & 3-4: adiabatically reversible (isentropic) Process 2–3 & 4-1: Heat transfer ∆T vanishingly small reversible; ∆T 0 Reservoir TH & TC const. gas T const. QH QC
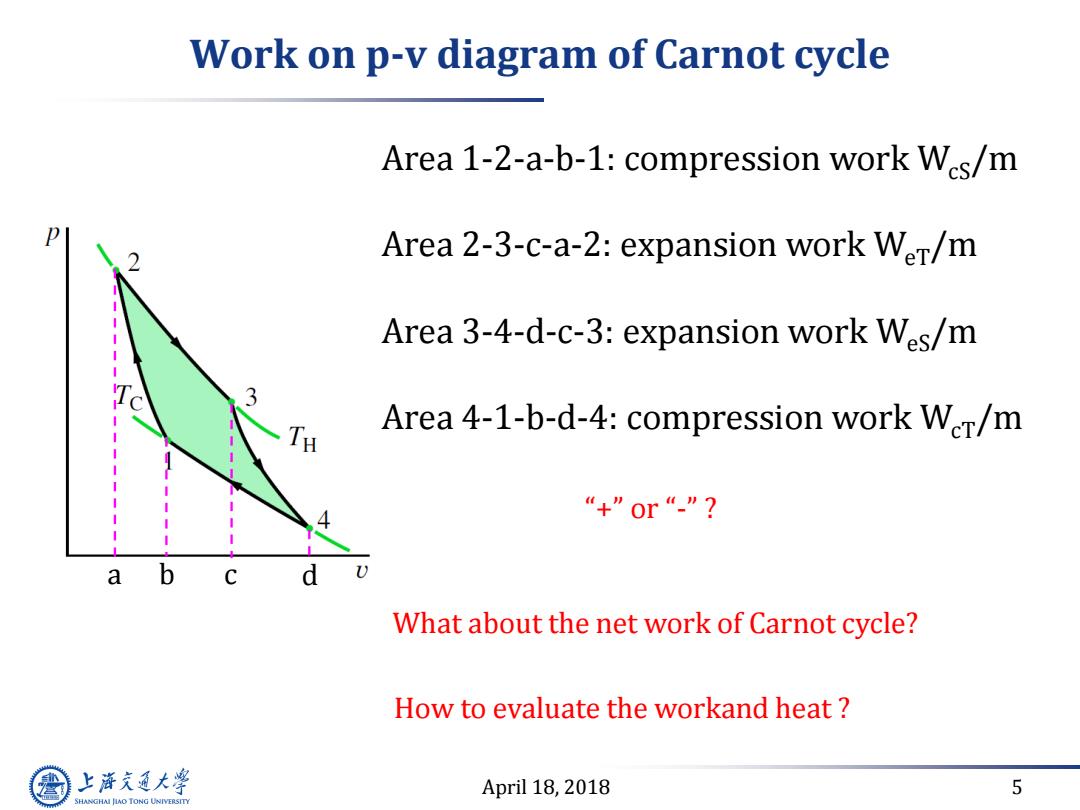
Work on p-v diagram of Carnot cycle Area 1-2-a-b-1:compression work Wcs/m Area 2-3-c-a-2:expansion work WeT/m Area 3-4-d-c-3:expansion work Wes/m Area 4-1-b-d-4:compression work WeT/m “+”0r“”? a d What about the net work of Carnot cycle? How to evaluate the workand heat 上游究通大学 April 18,2018 5 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 5 Work on p-v diagram of Carnot cycle Area 1-2-a-b-1: compression work WcS/m Area 2-3-c-a-2: expansion work WeT/m Area 3-4-d-c-3: expansion work WeS/m Area 4-1-b-d-4: compression work WcT/m a b c d What about the net work of Carnot cycle? How to evaluate the workand heat ? “+” or “-” ?
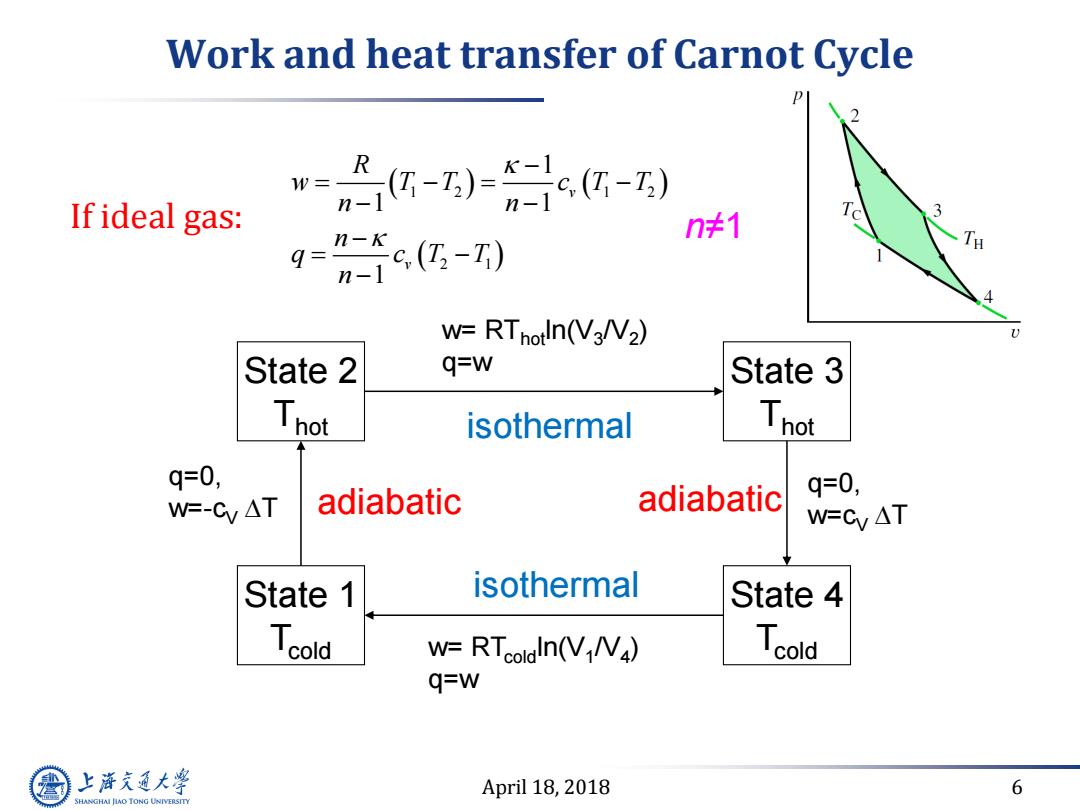
Work and heat transfer of Carnot Cycle w= If ideal gas: -)0c-) n-1 gg-) n≠1 W=RTnotln(V3NV2) State 2 q=w State 3 Thot isothermal Thot q=0, adiabatic adiabatic q=0, W=-Cy AT W=Cy AT State 1 isothermal State 4 Tcold W=RTcoldln(VN) Tcold q=w 上游充通大 April 18,2018 6 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 6 Work and heat transfer of Carnot Cycle State 2 Thot State 3 Thot State 1 Tcold State 4 Tcold isothermal adiabatic adiabatic isothermal q=0, w=-cV T q=0, w=cV T w= RThotln(V3 /V2 ) q=w w= RTcoldln(V1 /V4 ) q=w If ideal gas: 1 2 1 2 2 1 1 1 1 1 v v R w T T c T T n n n q c T T n n≠1

How to realize Carnot cycle Not limited to closed system in piston-cylinder also to vapor power plant (boiler,phase change,const.T) /boiler 4 3 _TH pump turbine TH condenser 、 Tc U also charge/discharge capacitor,magnetize/demagnetize paramagnetic substance,.. 上游充通大学 April 18,2018 7 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 7 How to realize Carnot cycle Not limited to closed system in piston-cylinder also to vapor power plant (boiler, phase change, const. T) turbine condenser pump boiler also charge/discharge capacitor, magnetize/ demagnetize paramagnetic substance, …

Reverse Carnot cycles Refrigeration/Heat pump cycle Process 1-2:gas expands isothermally at Shaded area:input work 1 Tc while receiving energy Qc from cold reservoir Process 2-3:gas compressed adiabatically 3 TH=const. until its temperature is TH. net.in Process 3-4:gas compressed isothermally at Ty while it discharges Te const. energy QH to hot reservoir. 2c 2 Process 4-1:gas expands adiabatically until its temperature V decreases to Tc. 图 上游充通大 April 18,2018 8 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 8 Reverse Carnot cycles Refrigeration / Heat pump cycle : Process 1–2: gas expands isothermally at TC while receiving energy QC from cold reservoir Process 2–3: gas compressed adiabatically until its temperature is TH . Process 3–4:gas compressed isothermally at TH while it discharges energy QH to hot reservoir. Process 4–1: gas expands adiabatically until its temperature decreases to TC . Shaded area: input work

Homework: Problems: C6.82,C6.105 Reading: Chap.C 7.1;or M5.11&M6.1 国上泽迁大学 April 18,2018 9 SHANGHAI JIAO TONG UNIVERSITY
April 18, 2018 9 Homework: Problems: C6.82, C6.105 Reading: Chap. C 7.1; or M5.11&M6.1