
上游充通大学 SHANGHAI JIAO TONG UNIVERSITY Engineering Thermodynamics I Lecture 22 Chapter 5 Mass and Energy Analysis of Control Volume Analysis (Section 5.5) Spring 2017 强 Prof.,Dr.Yonghua HUANG MAMLAMMAA http://cc.sjtu.edu.cn/G2S/site/thermo.html 1日
Engineering Thermodynamics I Lecture 22 Spring 2017 Prof., Dr. Yonghua HUANG Chapter 5 Mass and Energy Analysis of Control Volume Analysis http://cc.sjtu.edu.cn/G2S/site/thermo.html (Section 5.5)
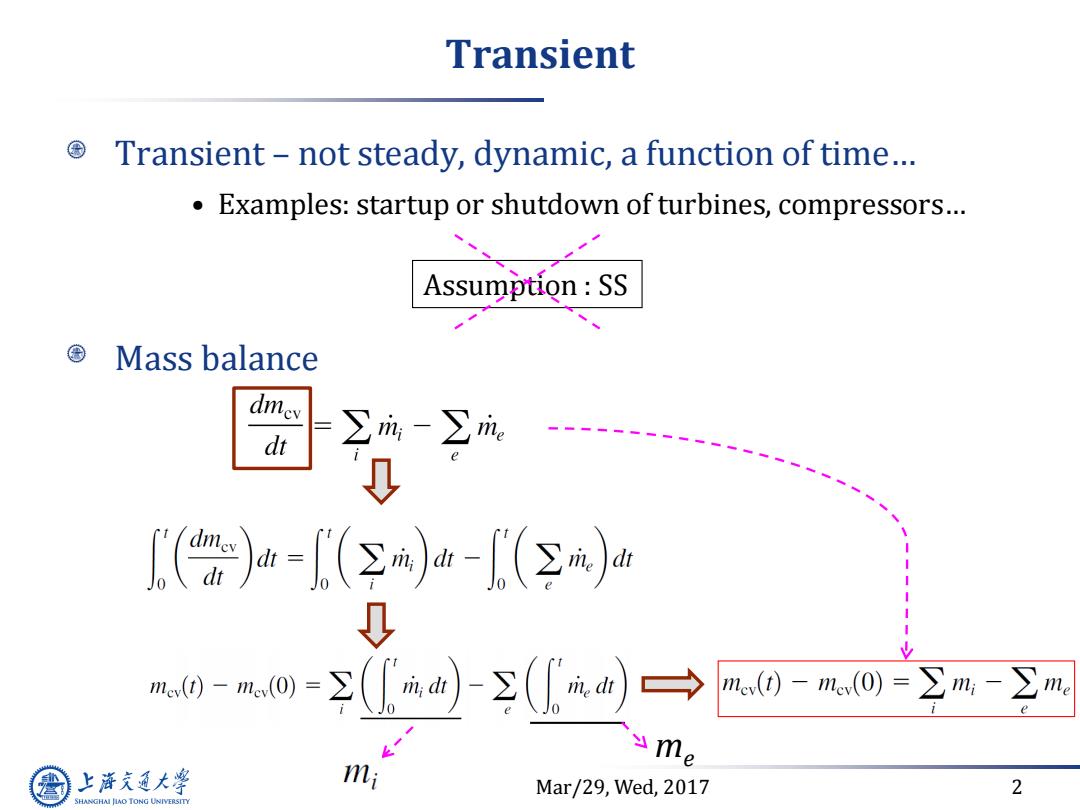
Transient Transient-not steady,dynamic,a function of time... Examples:startup or shutdown of turbines,compressors... Assumption:SS Mass balance dmev dt ∑m-∑m ↓ [〔()山-()血-(Σj血 R0-a0=①-①a)→ mev()-me(0)=∑n:-∑me me 上游通大学 mi Mar/29,Wed,2017 2 SHANGHAI JLAO TONG UNIVERSITY
Mar/29, Wed, 2017 2 Transient Transient – not steady, dynamic, a function of time… • Examples: startup or shutdown of turbines, compressors… Assumption : SS Mass balance me
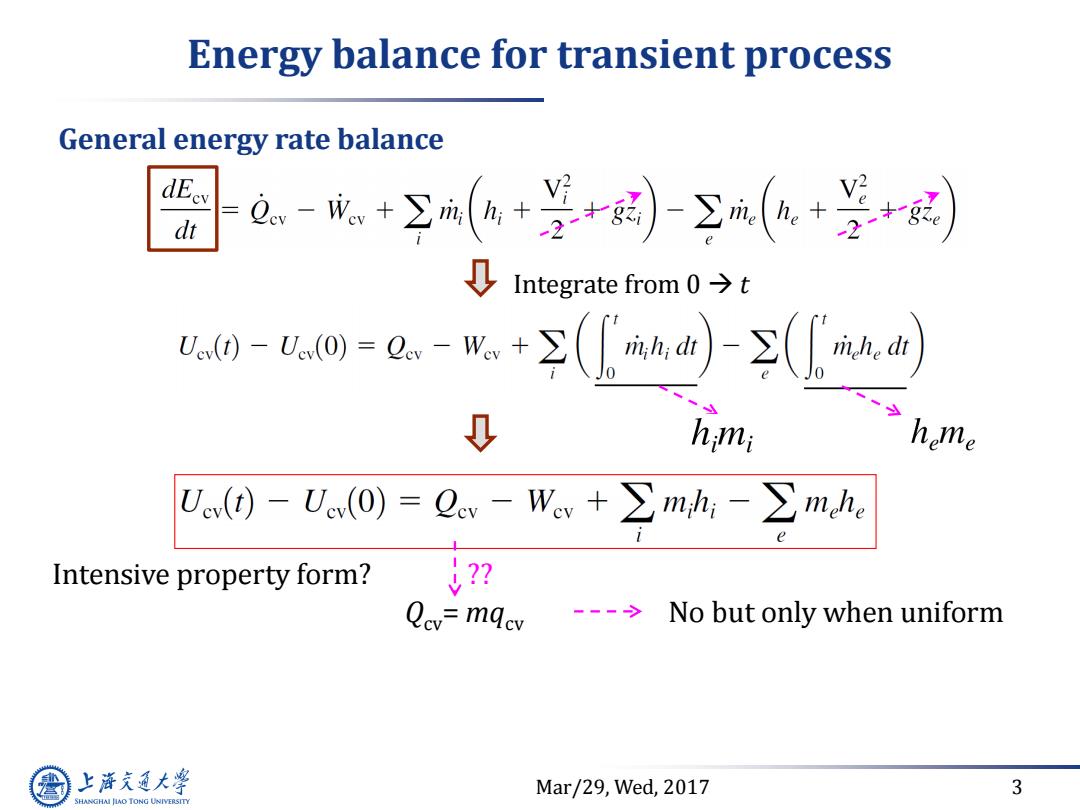
Energy balance for transient process General energy rate balance dEev dt a.-成+∑(a+年-到(+是+密 Integrate from 0>t 2m-o=0.-取+(a)-(a ↓ hmi home U(d)-Ue(O)=Oev-Wcv+∑mh;-∑mehe Intensive property form? --->No but only when uniform 上游充通大 Mar/29,Wed,2017 3 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 3 Energy balance for transient process General energy rate balance Integrate from 0 t Intensive property form? Qcv= mqcv No but only when uniform ??
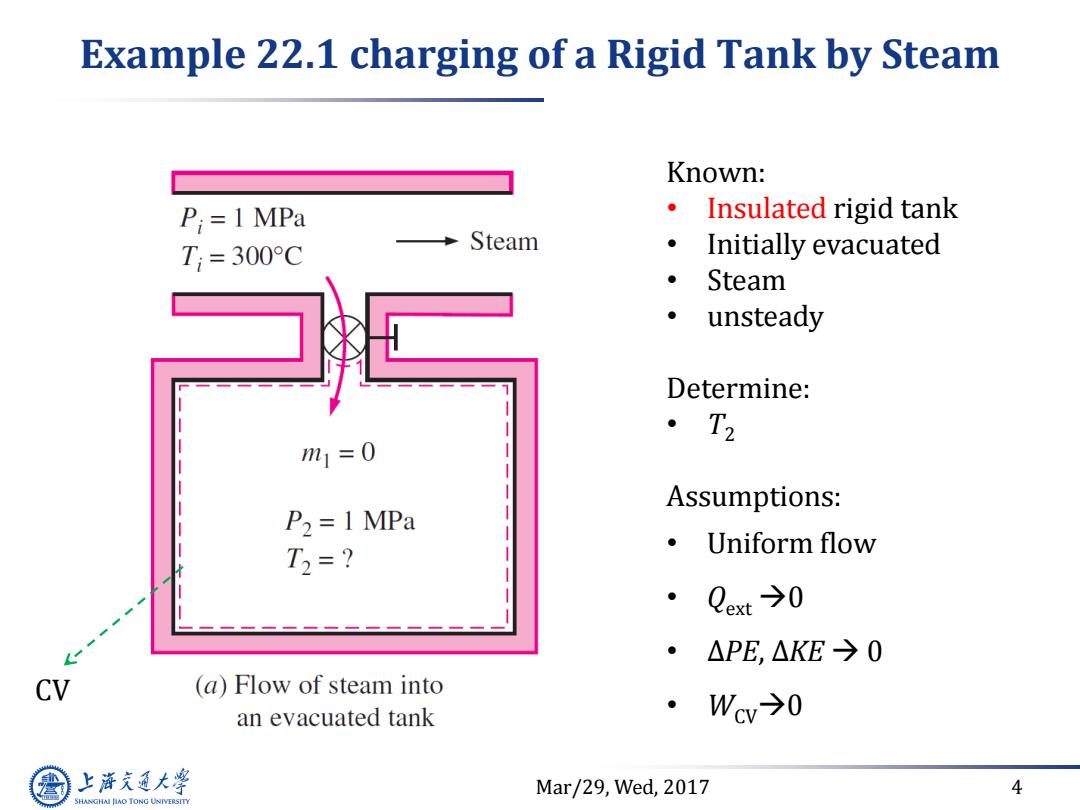
Example 22.1 charging of a Rigid Tank by Steam Known: P;=1 MPa ·Insulated rigid tank T;=300°C Steam Initially evacuated Steam unsteady Determine: ·T2 m1=0 Assumptions: P2 =1 MPa T2=? ·Uniform flow Qext→0 △PE,△KE→0 CV (a)Flow of steam into an evacuated tank ·Wv→0 上游文通大学 Mar/29,Wed,2017 4 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 4 Example 22.1 charging of a Rigid Tank by Steam Known: • Insulated rigid tank • Initially evacuated • Steam • unsteady Determine: • T2 Assumptions: • Uniform flow • Qext 0 • ∆PE, ∆KE 0 • WCV0 CV
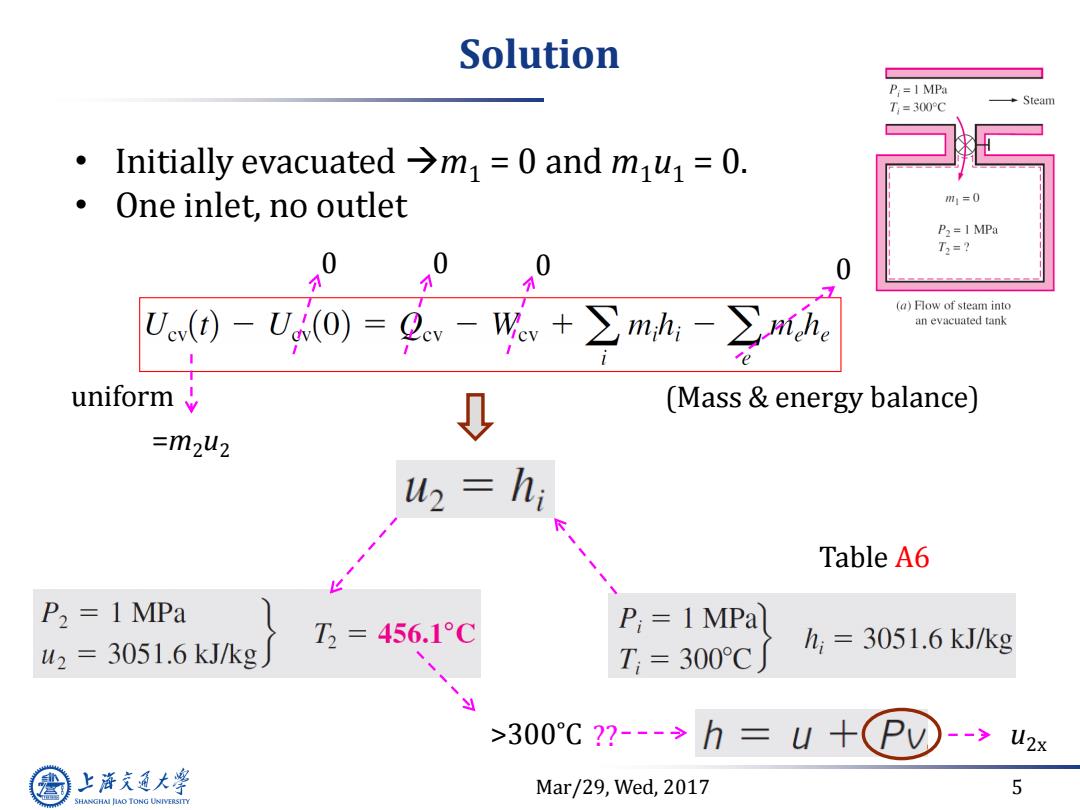
Solution P:=1 MPa T,=300°C Steam Initially evacuated >m=0 and mu=0. One inlet,no outlet m1=0 P2=I MPa T3=? 0 个 0 0 Un0-Uo)=Q-W,+∑mh-n, (a)Flow of steam into an evacuated tank uniform (Mass energy balance) =m2u2 u2=h; Table A6 P2 1 MPa T3=456.1°C P;=1MPa u2=3051.6 kJ/kg h=3051.6kJ/kg T,=300°C >300℃?--→h=U+ PV -->u2x 上游充通大 Mar/29,Wed,2017 5 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 5 Solution • Initially evacuated m1 = 0 and m1u1 = 0. • One inlet, no outlet 0 0 0 0 =m2u2 uniform Table A6 >300˚C u2x (Mass & energy balance) ??
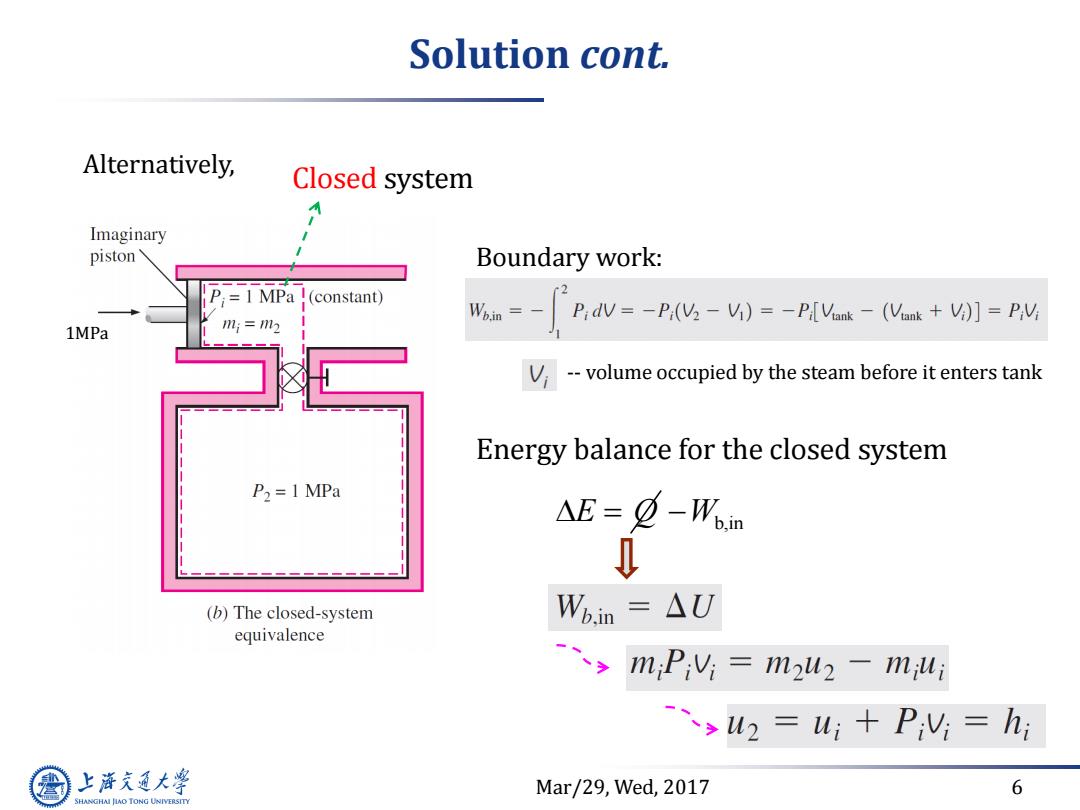
Solution cont. Alternatively, Closed system Imaginary piston' Boundary work: P;1 MPa !(constant) Pidv=-Pi(V2 -Vi)=-P[Vank-(Vink +Vi)]=PV; 1MPa ;=12 V--volume occupied by the steam before it enters tank Energy balance for the closed system P2=1 MPa △E=¢-Won (b)The closed-system Wbin=△U equivalence miPiVi=mu2-miui ->w2=u;+PV=h; 上游充通大 Mar/29,Wed,2017 6 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 6 Solution cont. Alternatively, Closed system 1MPa Boundary work: -- volume occupied by the steam before it enters tank Energy balance for the closed system E Q Wb,in

Example 22.2 Cooking with a Pressure Cooker Known: pressure regulator (petcock) ·Initially1 kg water 30 min Qin 1atm>p=75kPa(gage) 1atm 100 kPa ● unsteady System boundary. H20 m=I kg V=6L Determine: CV p=75 kPa(gage) Vapor e>Higher p, ·T2,m2 T(133C Liquid Assumptions: ·Uniform flow 0m=500W ·△PE,△KE→0 one exit and no inlets ·p(thus T)constant Steam leaves as a saturated vapor ·Wcy→0 上游充通大学 Mar/29,Wed,2017 7 SHANGHAI JLAO TONG UNIVERSITY
Mar/29, Wed, 2017 7 Example 22.2 Cooking with a Pressure Cooker Higher p, T (133˚C) pressure regulator (petcock) Known: • Initially 1kg water • 30 min Qin 1atmp=75kPa(gage) • 1atm = 100 kPa • unsteady Determine: • T2 , m2 Assumptions: • Uniform flow • ∆PE, ∆KE 0 • p (thus T) constant • Steam leaves as a saturated vapor • WCV0 CV one exit and no inlets

Solution Absolute pressure: System boundary. H2O Pabs Pgage Patm =75+100 =175 kPa m=I kg V=6L p=75 kPa (gage) Sat.steam leaving Vapor Liquid T=Tat@175kPa=116.04C Cin =500 W Mass energy balance: 0 U(④-U.O=Qv-W+∑ih:-∑m,h uniform =(m2u2-muj)cv -h.=么en5n=270.2Kkg Qin (m -m2)he (muz mu)cv =Qn△1=(0.5kJ/s)(30×60s)=900kJ 上泽通大学 Mar/29,Wed,2017 8 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 8 Solution Absolute pressure: Sat. steam leaving Mass & energy balance: 0 0 =(m2u2 - m1u1 )cv uniform 1
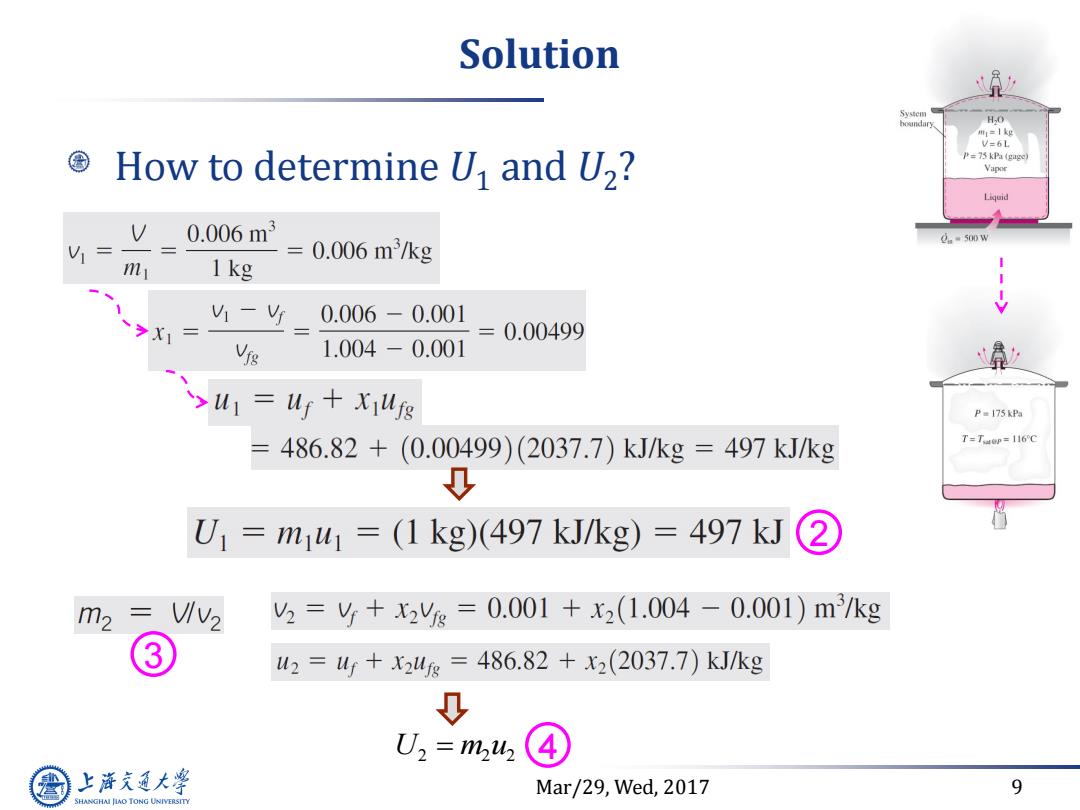
Solution System HO mr=1 kg V=6L How to determine U and U2? P=75 kPa (gage Vapor Liquid V0.006m3 0.006m3/kg 0.=500w = = m 1kg 1 - 0.006-0.001 0.00499 1.004-0.001 xu=uf+xiufs P =175 kPa = 486.82+(0.00499)(2037.7)kJ/kg=497kJ/kg T=TOp=116C 0 U1=m1w1=(1kg)(497kJ/kg)=497kJ ② m2 2=y+x2%=0.001+x2(1.004-0.001)m3/kg 3 42=4+24g=486.82+x2(2037.7)kJ/kg 0 U2=m2 上游充通大 Mar/29,Wed,2017 9 SHANGHAI JIAO TONG UNIVERSITY
Mar/29, Wed, 2017 9 Solution How to determine U1 and U2 ? U m u 2 2 2 2 3 4

Solution cont. ①②③④→ x2=0.009 2=0.001+(0.009)(1.004-0.001)m3/kg=0.010m3/kg V 0.006m3 m2 = 0.01m3/kg 0.6kg Note:that almost 12 water in the pressure cooker has evaporated during cooking 上游充通大学 Mar/29,Wed,2017 10 SHANGHAI JLAO TONG UNIVERSITY
Mar/29, Wed, 2017 10 Solution cont. 1 2 3 4 Note: that almost ½ water in the pressure cooker has evaporated during cooking