正在加载图片...
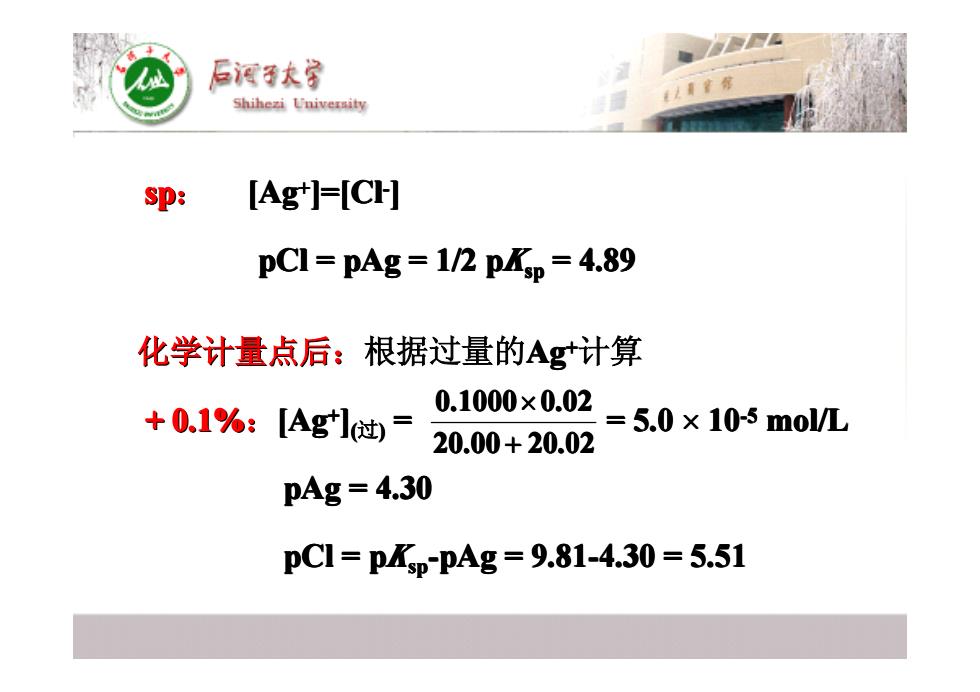
后酒子大宝 Shihezi University sp: [Ag']=[CH] pC1=pAg=1/2pp=4.89 化学计量点后:根据过量的Ag计算 +01%:Agl-0008-50x10smoM 20.00+20.02 pAg=4.30 pC1=PASp-PAg=9.81-4.30=5.51 sp: [Ag++ ]=[Cl -] pCl = pAg = 1/2 pKspsp = 4.89 化学计量点后: 化学计量点后:根据过量的Ag++计算 + 0.1%:[Ag++ ] ((过)) = = 5.0 = = 5.0 = = 5.0 = = 5.0 = = 5.0 = = 5.0 = = 5.0 = = 5.0 × 10-5-5 mol/L pAg = 4.30 = 4.30 = 4.30 = 4.30 = 4.30 = 4.30 = 4.30 = 4.30 pCl = pKspsp-pAg = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 = 9.81-4.30 = 5.51 20.00 20.02 0.1000 0.02 + ×