正在加载图片...
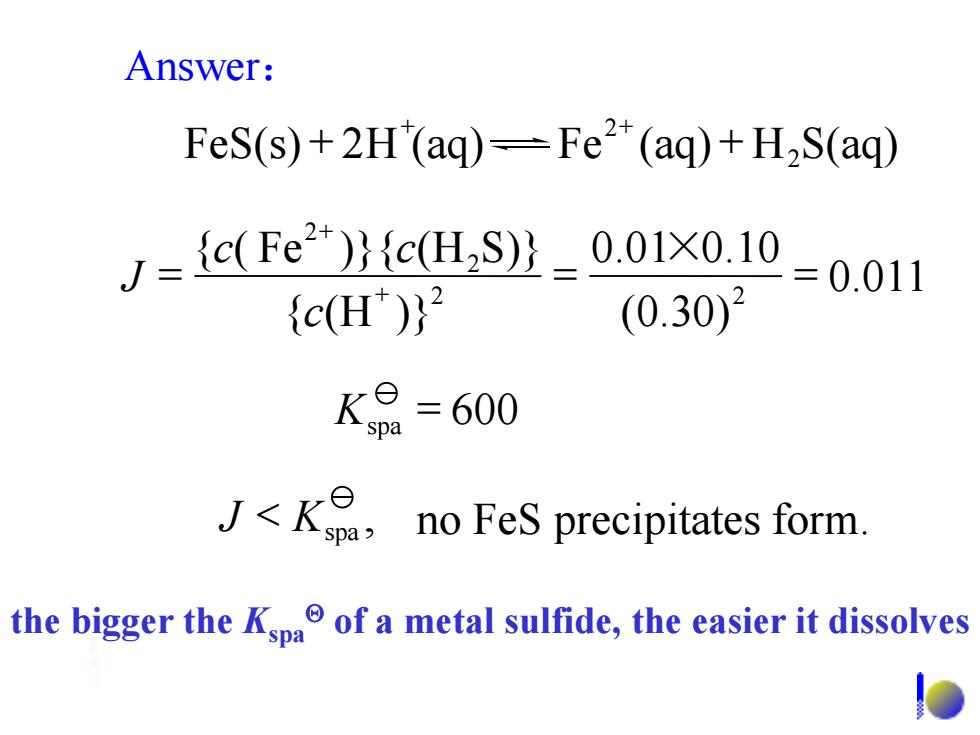
Answer: FeS(s)+2H(aq)=Fe2*(aq)+H2S(aq) J={c(Fe2)}{cH,Sy-0.01x0.1 {c(H)}2 (0.30)2 2=0.011 K8=600 J<K no FeS precipitates form. the bigger the Kpa of a metal sulfide,the easier it dissolves 0.011 (0.30) 0.01 0.10 { (H )} { ( Fe )}{ (H S)} 2 2 2 2 c c c J = × = = + + 2 + + + + Answer: FeS(s) 2H (aq) Fe (aq) H S(aq) 2 Kspa = 600 , no FeS precipitates form. J Kspa < the bigger the Kspa of a metal sulfide, the easier it dissolves