正在加载图片...
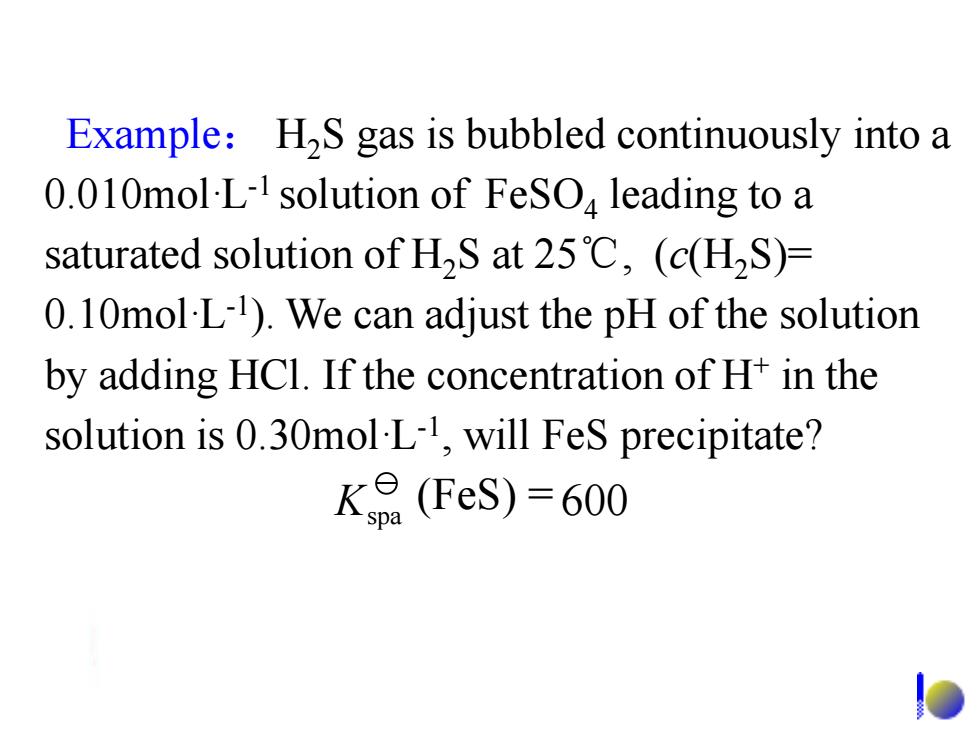
Example:H2S gas is bubbled continuously into a 0.010mol-L-1 solution of FeSO leading to a saturated solution of H2S at 25C,(c(H2S)= 0.10mol L1).We can adjust the pH of the solution by adding HCl.If the concentration of H+in the solution is 0.30mol L-1,will FeS precipitate? K9(FeS)=600Example: H2S gas is bubbled continuously into a 0.010mol·L-1 solution of FeSO4 leading to a saturated solution of H2S at 25℃, (c(H2S)= 0.10mol·L-1 ). We can adjust the pH of the solution by adding HCl. If the concentration of H+ in the solution is 0.30mol·L-1 , will FeS precipitate? Kspa (FeS) = 600