正在加载图片...
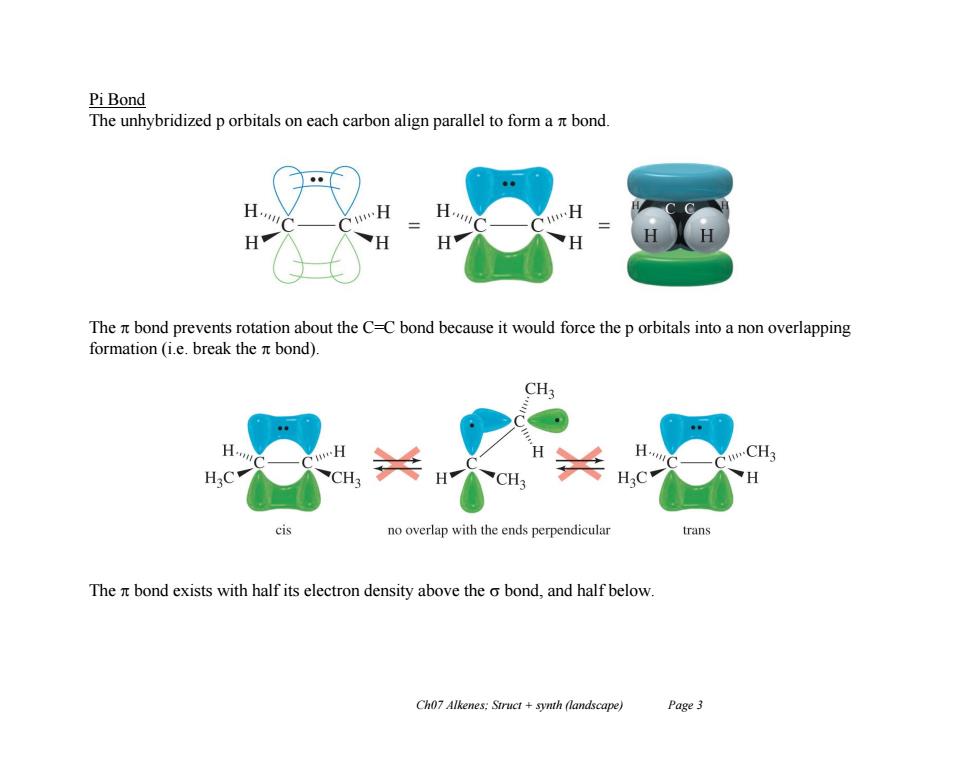
Pi Bond The unhybridized p orbitals on each carbon align parallel to form a nt bond. The n bond prevents rotation about the C-C bond because it would force the p orbitals into a non overlapping formation (i.e.break the nt bond). CH H CH3 CH CH no overlap with the ends perpendicular ran The nt bond exists with half its electron density above the o bond,and half below. Ch07 Alkenes:Struct +synth (landscape) Page3 Ch07 Alkenes; Struct + synth (landscape) Page 3 Pi Bond The unhybridized p orbitals on each carbon align parallel to form a bond. The bond prevents rotation about the C=C bond because it would force the p orbitals into a non overlapping formation (i.e. break the bond). The bond exists with half its electron density above the bond, and half below