正在加载图片...
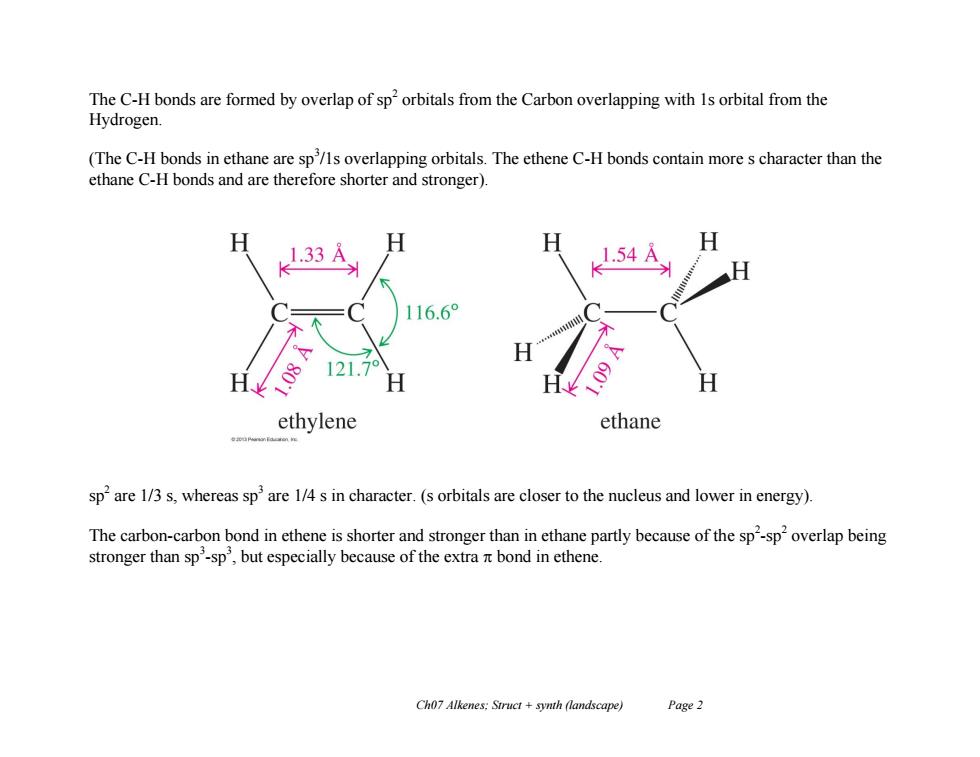
The C-H bonds are formed by overlap of sp'orbitals from the Carbon overlapping with 1s orbital from the Hydrogen. (The C-H bonds in ethane are sp/ls overlapping orbitals.The ethene C-H bonds contain more s character than the ethane C-H bonds and are therefore shorter and stronger). 1.33A H H 1.54A 116.6° 121.7 H ethylene ethane sp'are 1/3 s,whereas sp'are 1/4 s in character.(s orbitals are closer to the nucleus and lower in energy). The carbon-carbon bond in ethene is shorter and stronger than in ethane partly because of the sp'-sp overlap being stronger than sp'-sp',but especially because of the extra it bond in ethene. Ch07 Alkenes:Struct synth (landscape) Page 2Ch07 Alkenes; Struct + synth (landscape) Page 2 The C-H bonds are formed by overlap of sp2 orbitals from the Carbon overlapping with 1s orbital from the Hydrogen. (The C-H bonds in ethane are sp3 /1s overlapping orbitals. The ethene C-H bonds contain more s character than the ethane C-H bonds and are therefore shorter and stronger). sp 2 are 1/3 s, whereas sp3 are 1/4 s in character. (s orbitals are closer to the nucleus and lower in energy). The carbon-carbon bond in ethene is shorter and stronger than in ethane partly because of the sp 2 -sp 2 overlap being stronger than sp3 -sp 3 , but especially because of the extra bond in ethene