正在加载图片...
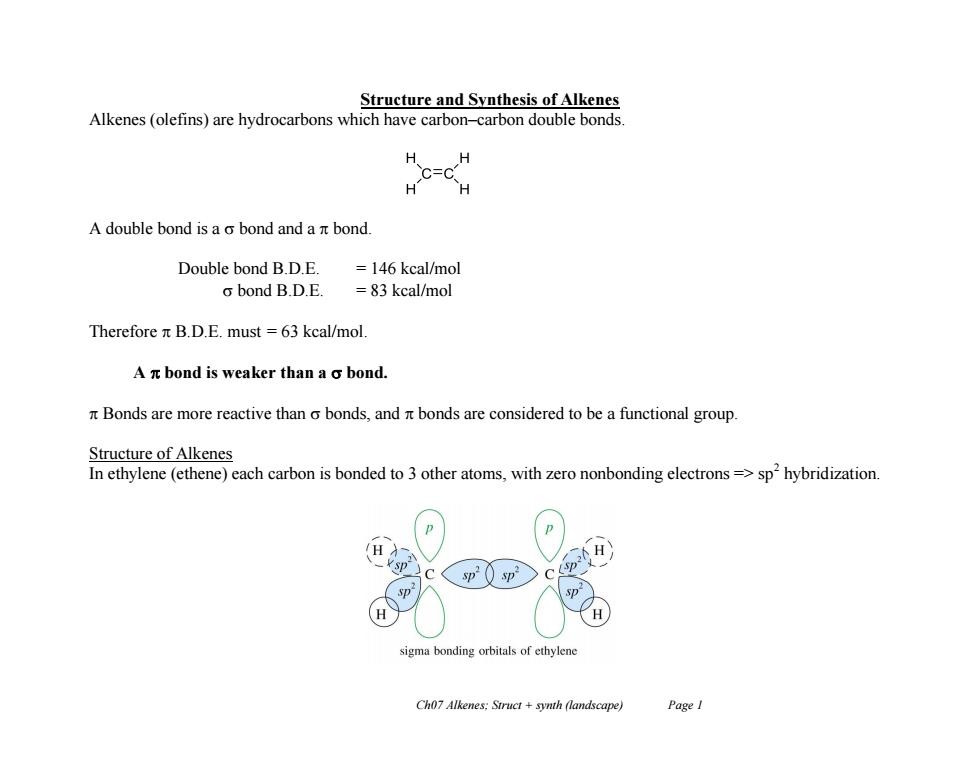
Structure and Synthesis of Alkenes Alkenes(olefins)are hydrocarbons which have carbon-carbon double bonds H H =C H- H A double bond is a o bond and a nt bond. Double bond B.D.E. =146 kcal/mol o bond B.D.E. =83 kcal/mol Therefore n B.D.E.must =63 kcal/mol. A n bond is weaker than a o bond. Bonds are more reactive than o bonds,and r bonds are considered to be a functional group. Structure of Alkenes In ethylene(ethene)each carbon is bonded to 3 other atoms,with zero nonbonding electrons=>sp'hybridization. sigma bonding orbitals of ethylene Ch07 Alkenes:Struct +synth (landscape) Page I Ch07 Alkenes; Struct + synth (landscape) Page 1 Structure and Synthesis of Alkenes Alkenes (olefins) are hydrocarbons which have carbon–carbon double bonds. A double bond is a bond and a bond. Double bond B.D.E. = 146 kcal/mol bond B.D.E. = 83 kcal/mol Therefore B.D.E. must = 63 kcal/mol. A bond is weaker than a bond. Bonds are more reactive than bonds, and bonds are considered to be a functional group. Structure of Alkenes In ethylene (ethene) each carbon is bonded to 3 other atoms, with zero nonbonding electrons => sp2 hybridization