正在加载图片...
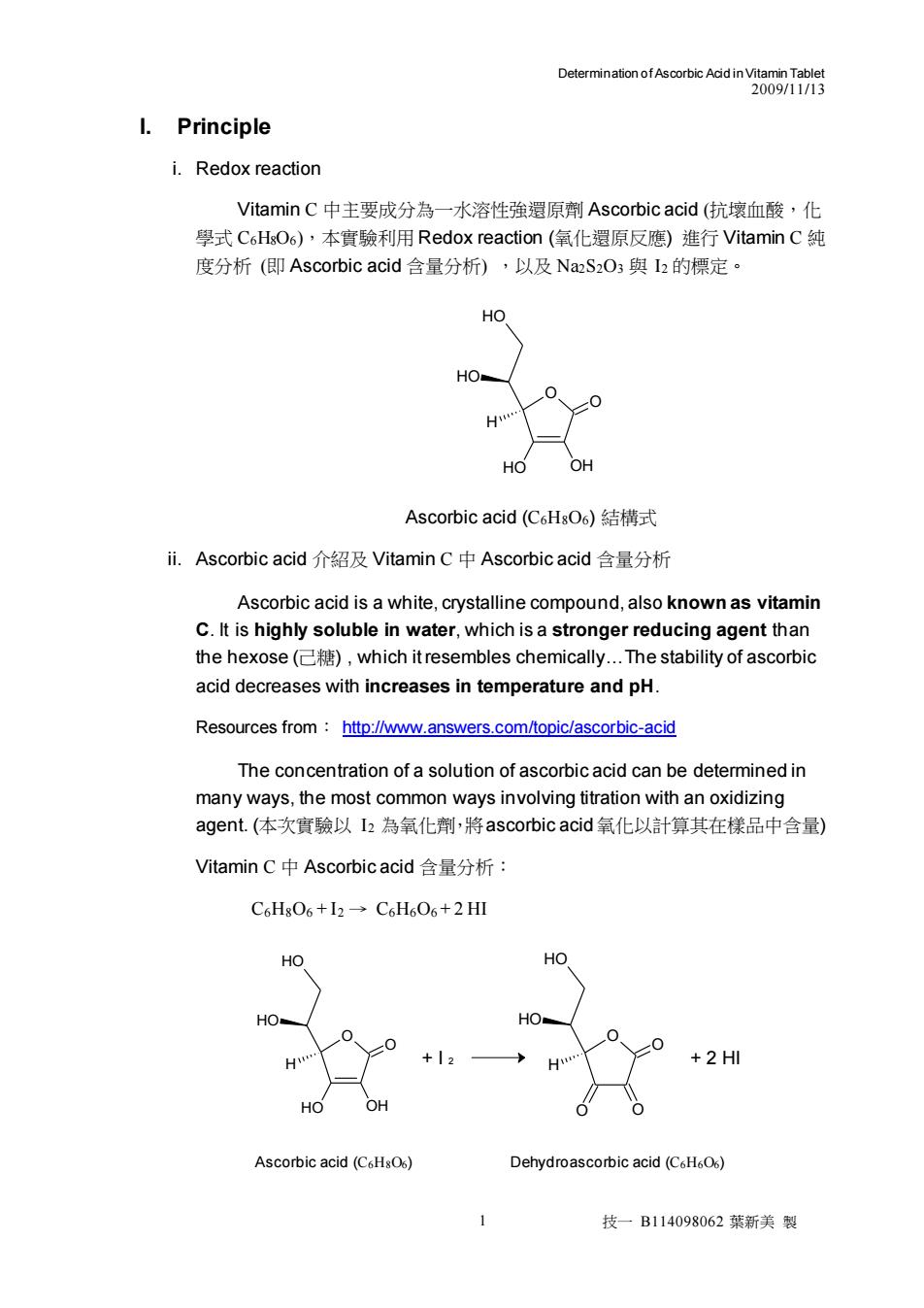
DetemnaionofecatcAadnv280gTte I.Principle i.Redox reaction Vitamin C中主要成分為一水溶性強還原劑Ascorbic acid(抗壞血酸,化 學式C6HsOs),本實驗利用Redox reaction(氧化還原反應)進行Vitamin C纯 度分析(即Ascorbic acid含量分析),以及NaS2O3與h的標定· HO H OH Ascorbic acid(C6HsO6)结構式 i,Ascorbic acid介绍及Vitamin C中Ascorbic acid含量分析 Ascorbic acid is a white,crystalline compound,also known as vitamin C.It is highly soluble in water,which is a stronger reducing agent than the hexose(),which it resembles chemically.The stability of ascorbic acid decreases with increases in temperature and pH. Resources from:http://www.answers.com/topic/ascorbic-acid The concentration of a solution of ascorbic acid can be determined in many ways,the most common ways involving titration with an oxidizing agent.(本次實驗以I2為氧化削,將ascorbic acid氧化以計算其在樣品中含量) Vitamin C中Ascorbic acid含量分析: C6HsO6+12-C6H606+2 HI HO HO Ascorbic acid (C.HsO) Dehydroascorbic acid(C.HO) 技一B114098062葉新美裂Determination of Ascorbic Acid in Vitamin Tablet 2009/11/13 1 技一 B114098062 葉新美 製 I. Principle i. Redox reaction Vitamin C 中主要成分為一水溶性強還原劑 Ascorbic acid (抗壞血酸,化 學式 C6H8O6),本實驗利用 Redox reaction (氧化還原反應) 進行 Vitamin C 純 度分析 (即 Ascorbic acid 含量分析) ,以及 Na2S2O3 與 I2 的標定。 O O HO OH HO HO H Ascorbic acid (C6H8O6) 結構式 ii. Ascorbic acid 介紹及 Vitamin C 中 Ascorbic acid 含量分析 Ascorbic acid is a white, crystalline compound, also known as vitamin C. It is highly soluble in water, which is a stronger reducing agent than the hexose (己糖) , which it resembles chemically.The stability of ascorbic acid decreases with increases in temperature and pH. Resources from: http://www.answers.com/topic/ascorbic-acid The concentration of a solution of ascorbic acid can be determined in many ways, the most common ways involving titration with an oxidizing agent. (本次實驗以 I2 為氧化劑,將ascorbic acid氧化以計算其在樣品中含量) Vitamin C 中 Ascorbic acid 含量分析: C6H8O6 + I2 → C6H6O6 + 2 HI O O O O HO HO H O O HO OH HO HO H + I 2 + 2 HI Ascorbic acid (C6H8O6) Dehydroascorbic acid (C6H6O6)