正在加载图片...
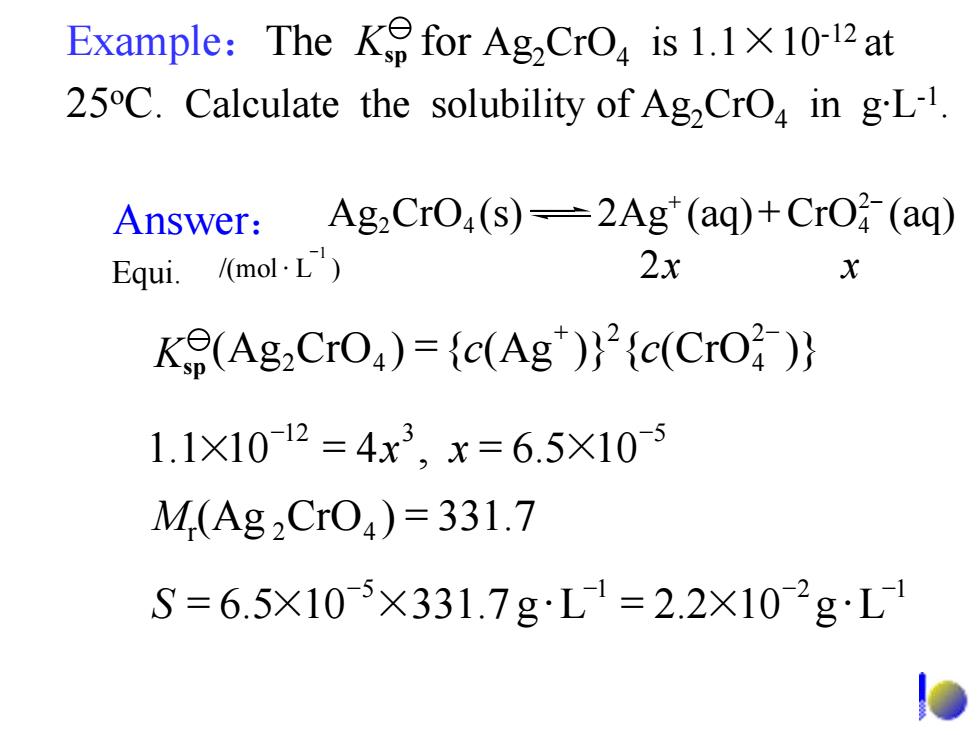
Example:The Ke for AgCrO is 1.1X10-12at 25C.Calculate the solubility of Ag,CrO in gL-1. Answer:Ag2 CrO,(s)-2Ag"(aq)+CrO(aq) Equi. /(mol.L) 2x X Ke(Ag2CrO)=(c(Ag)(c(CrO 1.1X1012=4x3,x=6.5×10-5 M(Ag2Cr04)=331.7 S=6.5X10-5×331.7gL1=2.2X102gL /(mol )L 2 1 xx − Equi. ⋅ Mr(Ag 42 )CrO = 331.7 5 1 12 5.6 10 331.7 2.2Lg 10 Lg − − −− S = ×× =⋅ × ⋅ 12 3 5 1.1 10 5.6,4 10 − − × xx == × Ag CrO 2Ag(s) (aq) (CrO aq) 42 4 + 2− + + 2− 4 2 42 K (Ag = ({)CrO Ag )} cc CrO({ )} sp Answer: Example:The for Ag2CrO4 is 1.1×10-12 at 25oC. Calculate the solubility of Ag2CrO4 in g·L-1. Ksp