正在加载图片...
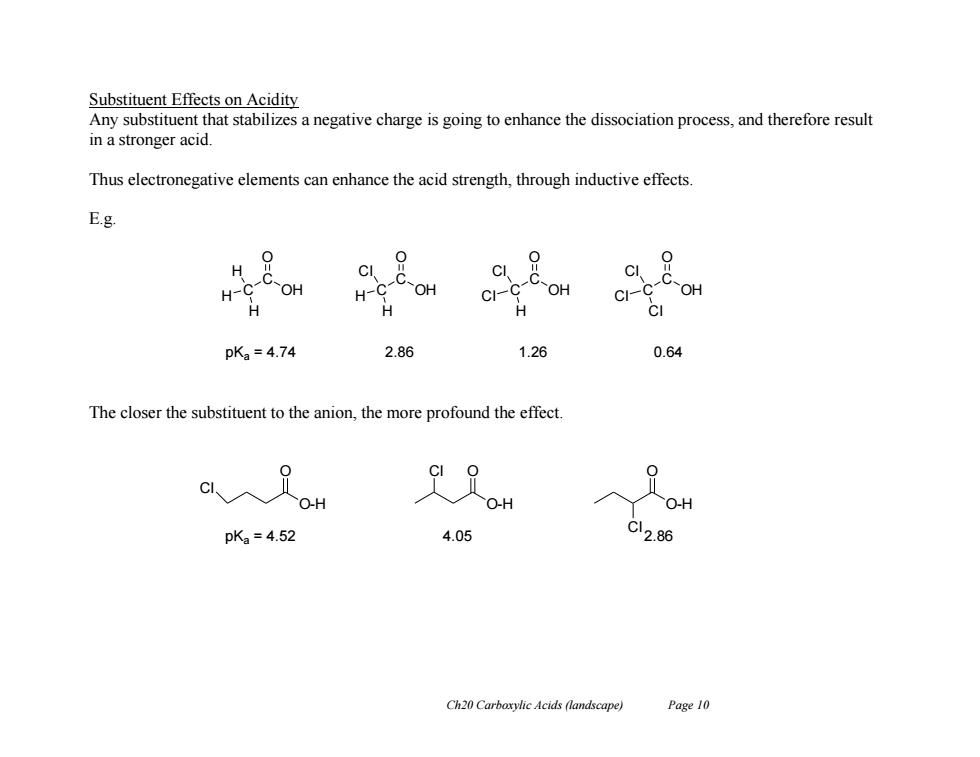
Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process,and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength,through inductive effects. E.g. H 9 0 C OH C CI H- H-C OH CI-C OH ci-4 C-OH H H H pKa=4.74 2.86 1.26 0.64 The closer the substituent to the anion,the more profound the effect OH OH O-H pKa=4.52 4.05 C12.86 Ch20 Carboxylic Acids (landscape) Page 10 Ch20 Carboxylic Acids (landscape) Page 10 Substituent Effects on Acidity Any substituent that stabilizes a negative charge is going to enhance the dissociation process, and therefore result in a stronger acid. Thus electronegative elements can enhance the acid strength, through inductive effects. E.g. The closer the substituent to the anion, the more profound the effect. C C OH O H H H C C OH O H Cl H C C OH O Cl Cl H C C OH O Cl Cl Cl pKa = 4.74 2.86 1.26 0.64 O-H O Cl O-H O O-H Cl O Cl pKa = 4.52 4.05 2.86