正在加载图片...
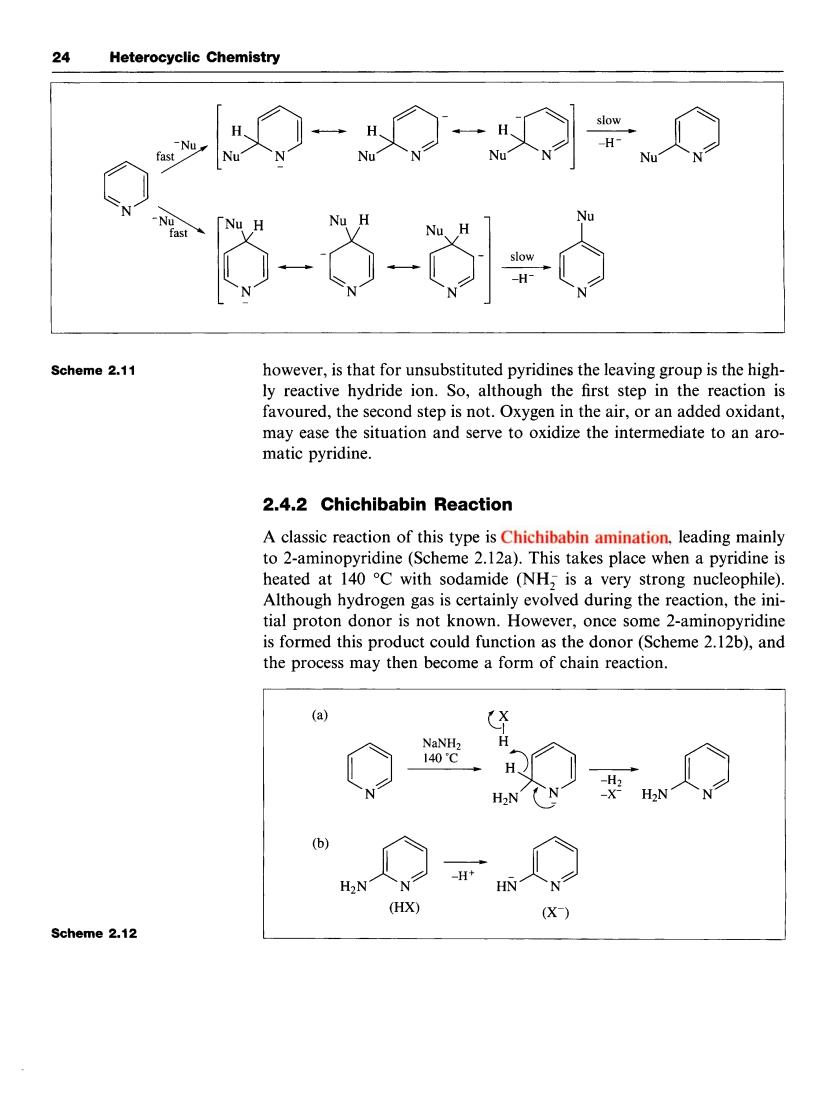
24 Heterocyclic Chemistry 一◇一÷◇ 3-的d Scheme 2.11 however,is that for unsubstituted pyridines the leaving group is the high ly reactive hydride ion.So,although the first step in the reaction is favoured,the second step is not.Oxygen in the air,or an added oxidant, may ease the situation and serve to oxidize the intermediate to an aro- matic pyridine. 2.4.2 Chichibabin Reaction A classic reaction of this type is Chichibabin amination.leading mainly to 2-aminopyridine(Scheme 2.12a).This takes place when a pyridine is heated at 140 C with sodamide (NH,is a very strong nucleophile) Although hydrogen gas is certainly evolved during the reaction,the ini- tial proton donor is not known.However,once some 2-aminopyridin is formed this product could function as the donor(Scheme 2.12b),and the process may then become a form of chain reaction. (a H2N(N b HX (X) Scheme2.12 24 Heterocyclic Chemistry 0 N Nu Nu H Nu H N N N N Scheme 2.11 Scheme 2.12 however, is that for unsubstituted pyridines the leaving group is the highly reactive hydride ion. So, although the first step in the reaction is favoured, the second step is not. Oxygen in the air, or an added oxidant, may ease the situation and serve to oxidize the intermediate to an aromatic pyridine. 2.4.2 Chichibabin Reaction A classic reaction of this type is Chichibabin amination, leading mainly to 2-aminopyridine (Scheme 2.12a). This takes place when a pyridine is heated at 140 "C with sodamide (NH, is a very strong nucleophile). Although hydrogen gas is certainly evolved during the reaction, the initial proton donor is not known. However, once some 2-aminopyridine is formed this product could function as the donor (Scheme 2.12b), and the process may then become a form of chain reaction. 0 N