正在加载图片...
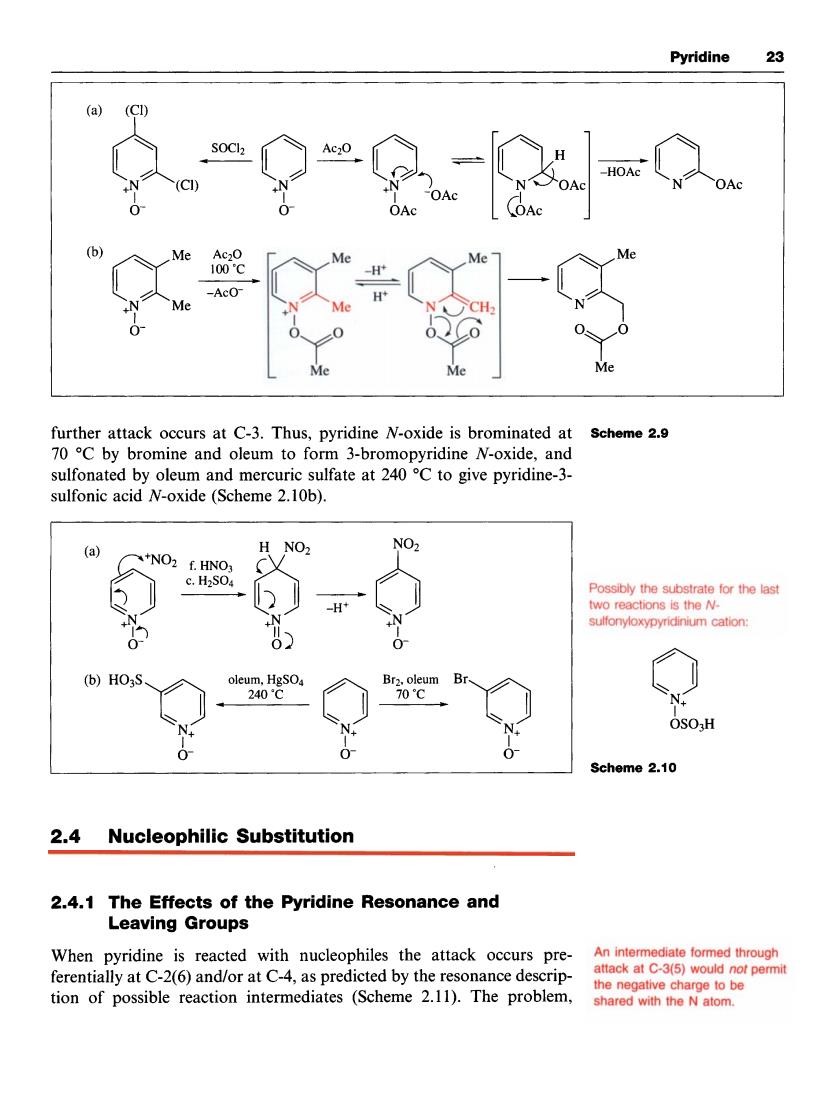
Pyridine 品 a (CI “92=0 further attack occurs at C-3.Thus,pyridine N-oxide is brominated at scheme 2.9 70 C by bromine and oleum to form 3-bromopyridine N-oxide,and sulfonated by oleum and mercuric sulfate at 240C to give pyridine-3- sulfonic acid N-oxide(Scheme 2.10b). H NO2 NO HNO 0 b)HO3 OSOH Scheme 2.10 24 Nucleophilic Substitution 2.4.1 The Effects of the Pyridine Resonance and Leaving Groups When pyridine is reacted with nucleophiles the attack occurs pre- ferentially at C-2(6)and/or at C-4,as predicted by the resonance descrip- he ne tion of possible reaction intermediates(Scheme 2.11).The probem shared with the N atom.Pyridine 23 further attack occurs at C-3. Thus, pyridine N-oxide is brominated at 70 "C by bromine and oleum to form 3-bromopyridine N-oxide, and sulfonated by oleum and mercuric sulfate at 240 "C to give pyridine-3- sulfonic acid N-oxide (Scheme 2. lob). Scheme 2.9 (a) H NO2 Possibly the substrate for the last two reactions is the Nsulfonyloxypyridinium cation: 0- 03 0- oleum, 240°C HgS04 0 Br;lp Brm ____c OS03H N+ I N+ I 0- 0- 0- Scheme 2.10 2.4 Nucleophilic Substitution 2.4.1 The Effects of the Pyridine Resonance and Leaving Groups An intermediate formed through attack at c-3(5) the negative charge to be When pyridine is reacted with nucleophiles the attack occurs preferentially at C-2(6) and/or at c-4, as predicted by the resonance descripnot permit tion of possible reaction intermediates (Scheme 2.1 1). The problem, sharedwith the Natom