正在加载图片...
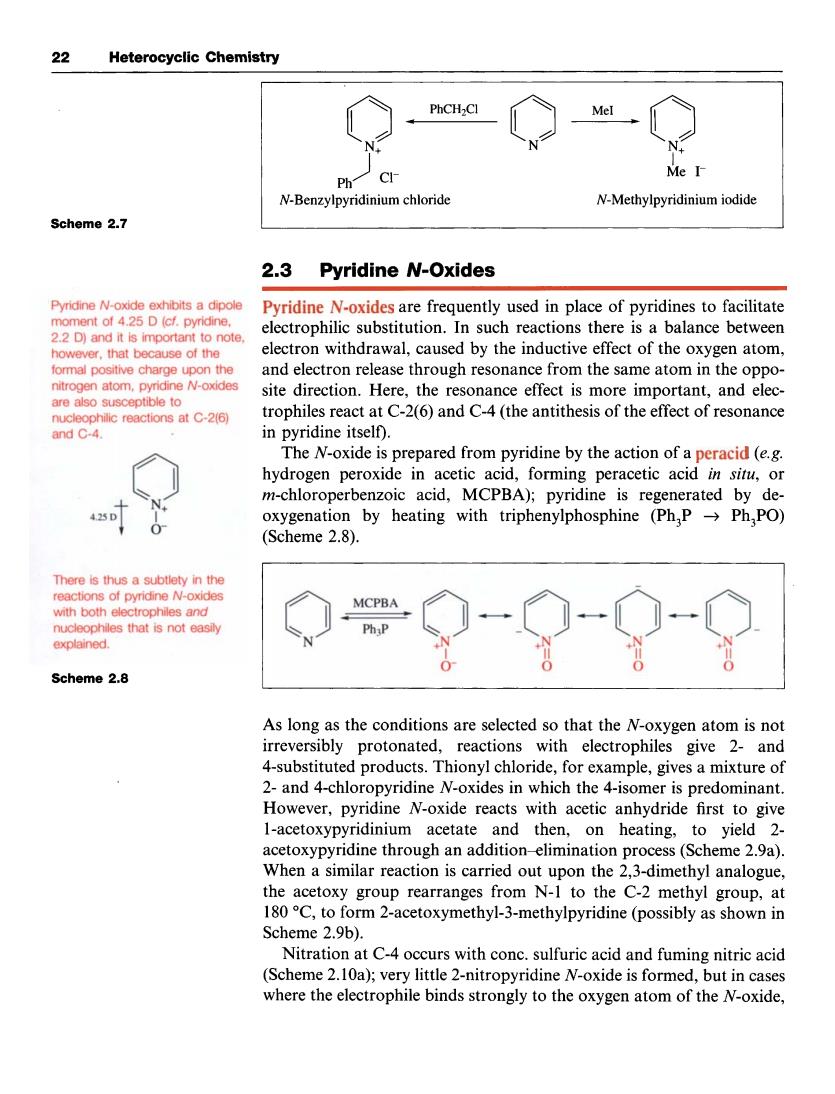
22 Heterocyclic Chemistry CI Me I N-Methylpyridinium iodide Scheme 2.7 2.3 Pyridine N-Oxides Pyridine N-oxides are frequently used in place of pyridines to facilitate 2.2 D)and it is important to note. electrophilic substitution.In such reactions there is a balance between however.that because of the o withdrawal,caused by the inductiveec of thxygen atom. and elease throuh resonance from the same atom in the oppo site direction.Here,the resonance effect is more important,and elec- trophiles react at C-2(6)and C-4 (the antithesis of the effect of resonance in pyridine itself). The N-oxide is prepared from pyridine by the action of a peracid(e.g hydrogen peroxide in acetic acid,forming peracetic acid in situ,or m-chloroperbenzoic acid,MCPBA);pyridine is regenerated by de- 0 oxygenation by heating with triphenylphosphine (Ph,PPh,PO) (Scheme 2.8). There is thus a subtlety in the tions of pyridine N-ox es that is not e explained Scheme 2.8 As long as the conditions are selected so that the N-oxygen atom is not irreversibly protonated,reactions with electrophiles give 2-and 4-substituted products.Thionyl chloride,for example,gives a mixture of 2-and 4-chloropyridine N-oxides in which the 4-is spredominant. However,pyridine N-oxide reacts with acetic anhydride first to give 1-acetoxypyridinium acetate and then,on heating,to yield 2- acetoxypyridine through an addition-elimination process(Scheme 2.9a) Whena similar eaction is carried out upo the23-dimethyl the acetoxy group rearranges from N-I to the C-2 methyl group,at 180C,to form 2-acetoxymethyl-3-methylpyridine(possibly as shown in Scheme 2.9b). Nitration at C-4 occurs with conc.sulfuric acid and fuming nitric acid (Scheme 2.10a);very little 2-nitropyridine N-oxide is formed,but in cases where the electrophile binds strongly to the oxygen atom of the N-oxide, 22 Heterocyclic Chemistry Scheme 2.7 Pyridine N-oxide exhibits a dipole moment of 4.25 D (cf. pyridine, 2.2 D) and it is important to note, however, that because of the formal positive charge upon the nitrogen atom, pyridine N-oxides are also susceptible to nucleophilic reactions at C-2(6) and C-4. $- 0- 0 OLIrJ - PhCH2C1 N N+ 1 N+ Me I- Ph I c1- N-Benzylpyridinium chloride N-Methylpyridinium iodide 2.3 Pyridine NlOxides Pyridine N-oxides are frequently used in place of pyridines to facilitate electrophilic substitution. In such reactions there is a balance between electron withdrawal, caused by the inductive effect of the oxygen atom, and electron release through resonance from the same atom in the opposite direction. Here, the resonance effect is more important, and electrophiles react at C-2(6) and C-4 (the antithesis of the effect of resonance in pyridine itself). The N-oxide is prepared from pyridine by the action of a peracid (e.g. hydrogen peroxide in acetic acid, forming peracetic acid in situ, or rn-chloroperbenzoic acid, MCPBA); pyridine is regenerated by deoxygenation by heating with triphenylphosphine (Ph,P + Ph,PO) (Scheme 2.8). There is thus a subtlety in the reactions of pyridine N-oxides with both electrophiles and nucleophiles that is not easily explained. Scheme 2.8 As long as the conditions are selected so that the N-oxygen atom is not irreversibly protonated, reactions with electrophiles give 2- and 4-substituted products. Thionyl chloride, for example, gives a mixture of 2- and 4-chloropyridine N-oxides in which the 4-isomer is predominant. However, pyridine N-oxide reacts with acetic anhydride first to give 1-acetoxypyridinium acetate and then, on heating, to yield 2- acetoxypyridine through an addition-elimination process (Scheme 2.9a). When a similar reaction is carried out upon the 2,3-dimethyl analogue, the acetoxy group rearranges from N-1 to the C-2 methyl group, at 180 "C, to form 2-acetoxymethyl-3-methylpyridine (possibly as shown in Scheme 2.9b). Nitration at C-4 occurs with conc. sulfuric acid and fuming nitric acid (Scheme 2.1Oa); very little 2-nitropyridine N-oxide is formed, but in cases where the electrophile binds strongly to the oxygen atom of the N-oxide