正在加载图片...
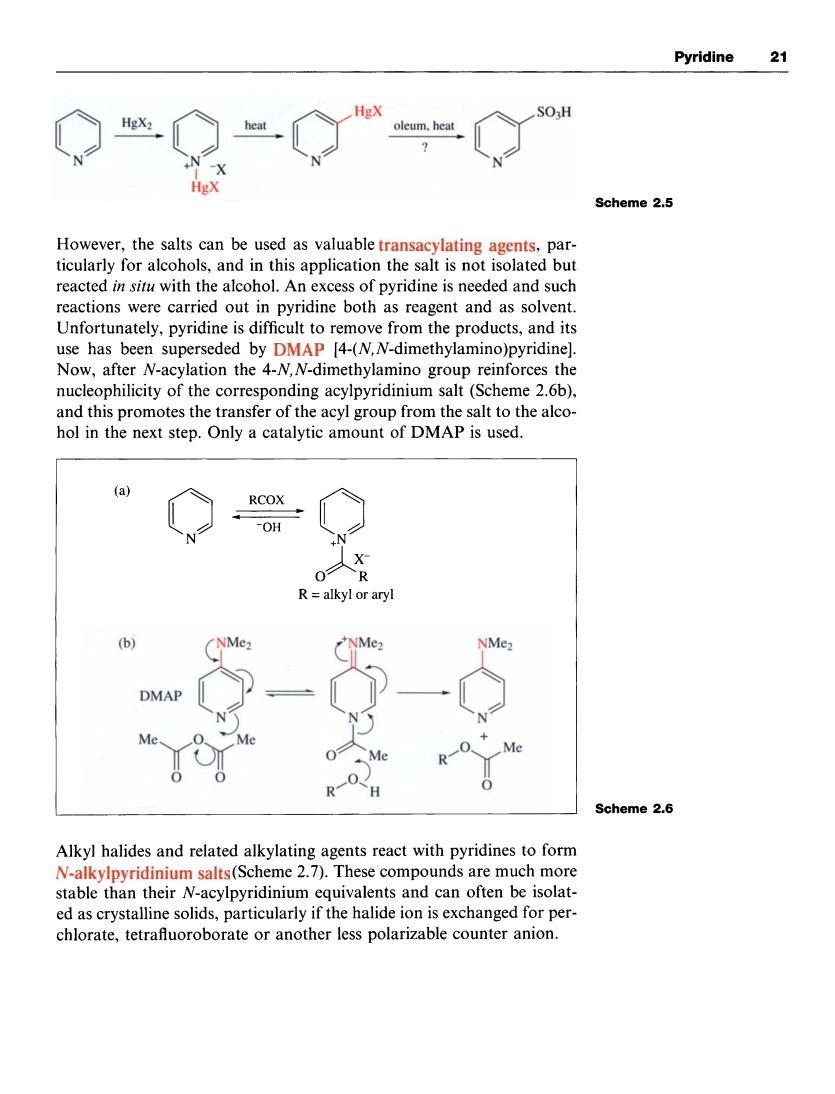
Pyridine 21 HgX: Scheme 2.5 However,the salts can be used as valuable transacylating agents,par- ticularly for alcohols,and in this application the salt is not isolated but reacted in situ with the alcohol.An excess of pyridine is needed and such tel riine s o rmofrom thepdt were carried out in pyridine both as reagent and as solvent use has been superseded by DMAP [4-(N,N-dimethylamino)pyridine] Now,after N-acylation the 4-N,N-dimethylamino group reinforces the of the responding acylpyridinium salt(). and this promotes the transfer of the acyl group from the salt to the alco hol in the next step.Only a catalytic amount of DMAP is used. RCOX -OH R=alkyl or aryl (b) NMe NMe. DMAP Me. Scheme 2.6 Alkyl halides and related alkylating agents react with pyridines to form N-alkylpyridinium salts(Scheme 2.7).These compounds are much more stable than their N-acylpyridinium equivalents and can often be isolat- ed as crystalline solids,particularly if the halide ion is exchanged for r per chlorate,tetrafluoroborate or another less polarizable counter anion.Pyridine 21 HgX Scheme 2.5 However, the salts can be used as valuable transacylating agents, particularly for alcohols, and in this application the salt is not isolated but reacted in situ with the alcohol. An excess of pyridine is needed and such reactions were carried out in pyridine both as reagent and as solvent. Unfortunately, pyridine is difficult to remove from the products, and its use has been superseded by DMAP [4-(N, N-dimethy1amino)pyridinel. Now, after N-acylation the 4-N, N-dimethylamino group reinforces the nucleophilicity of the corresponding acylpyridinium salt (Scheme 2.6b), and this promotes the transfer of the acyl group from the salt to the alcohol in the next step. Only a catalytic amount of DMAP is used. R = alkyl or aryl + R,oYMe 0 Scheme 2.6 Alkyl halides and related alkylating agents react with pyridines to form N-alkylpyridinium salts (Scheme 2.7). These compounds are much more stable than their N-acylpyridinium equivalents and can often be isolated as crystalline solids, particularly if the halide ion is exchanged for perchlorate, tetrafluoroborate or another less polarizable counter anion