正在加载图片...
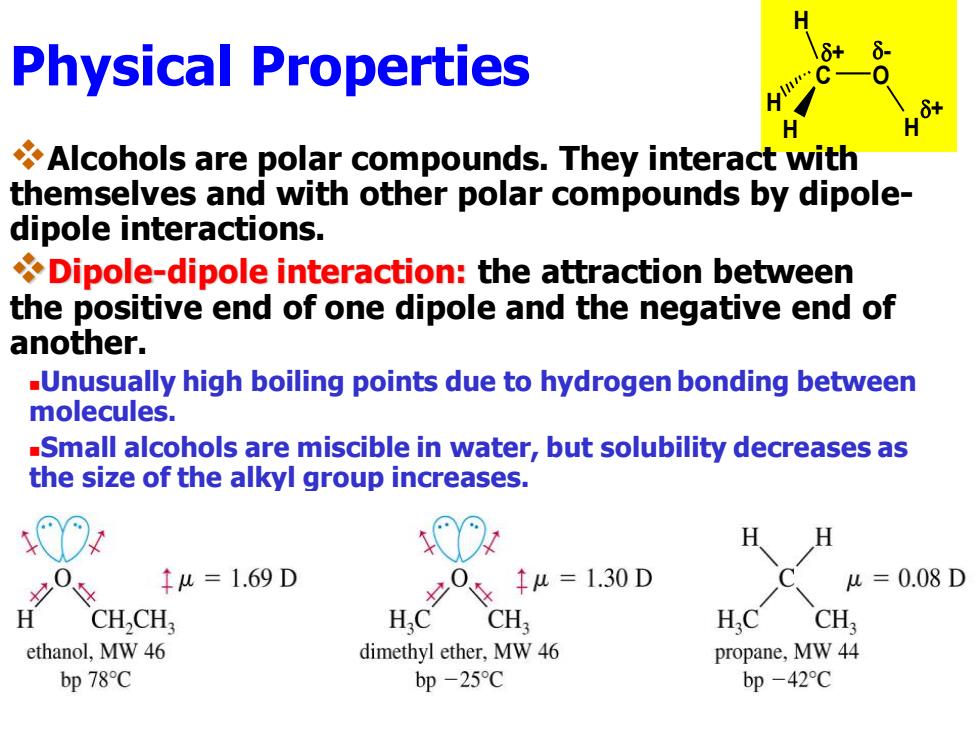
Physical Properties H Alcohols are polar compounds.They interact with themselves and with other polar compounds by dipole- dipole interactions. Dipole-dipole interaction:the attraction between the positive end of one dipole and the negative end of another. -Unusually high boiling points due to hydrogen bonding between molecules. -Small alcohols are miscible in water,but solubility decreases as the size of the alkyl group increases. 00 H H 0 1u=1.69D 0=1.30D u=0.08D H CH,CH, HC CH H.C CH ethanol,MW 46 dimethyl ether,MW 46 propane,MW 44 bp78℃ bp-25C bp-42CPhysical Properties - + + O H H H C H ❖Alcohols are polar compounds. They interact with themselves and with other polar compounds by dipoledipole interactions. ❖Dipole-dipole interaction: the attraction between the positive end of one dipole and the negative end of another. ◼Unusually high boiling points due to hydrogen bonding between molecules. ◼Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases