正在加载图片...
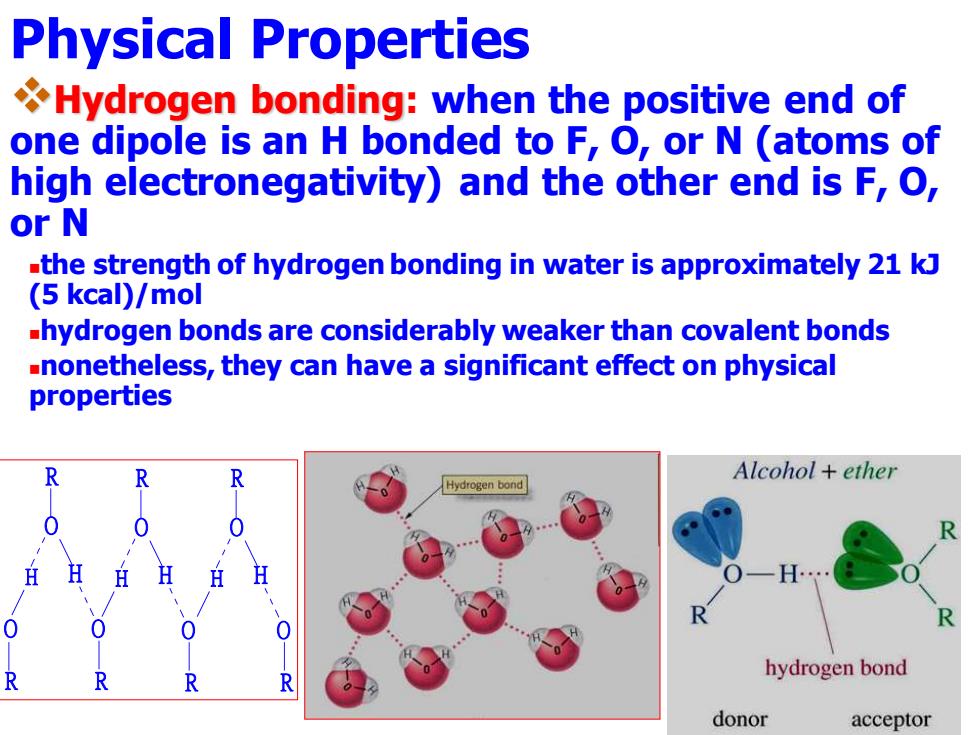
Physical Properties Hydrogen bonding:when the positive end of one dipole is an H bonded to F,O,or N (atoms of high electronegativity)and the other end is F,O, or N -the strength of hydrogen bonding in water is approximately 21 kJ (5 kcal)/mol hydrogen bonds are considerably weaker than covalent bonds -nonetheless,they can have a significant effect on physical properties Alcohol ether ydrogen bond R hydrogen bond donor acceptor Physical Properties ❖Hydrogen bonding: when the positive end of one dipole is an H bonded to F, O, or N (atoms of high electronegativity) and the other end is F, O, or N ◼the strength of hydrogen bonding in water is approximately 21 kJ (5 kcal)/mol ◼hydrogen bonds are considerably weaker than covalent bonds ◼nonetheless, they can have a significant effect on physical properties R O H H O O R R R O H H O R R O H H O R