正在加载图片...
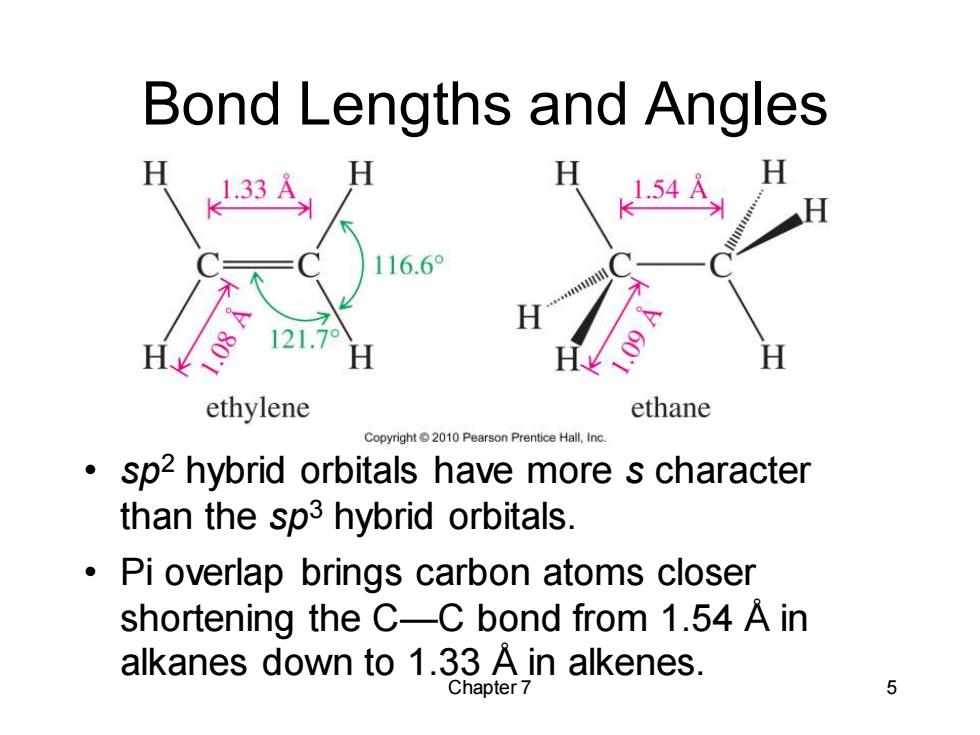
Bond Lengths and Angles 116.6° 121.7° H ethylene ethane Copyright2010 Pearson Prentice Hall,Inc. sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. Pi overlap brings carbon atoms closer shortening the C-C bond from 1.54 A in alkanes down to 1.33 A in alkenes. Chapter 7 5Chapter 7 5 Bond Lengths and Angles • sp2 hybrid orbitals have more s character than the sp3 hybrid orbitals. • Pi overlap brings carbon atoms closer shortening the C—C bond from 1.54 Å in alkanes down to 1.33 Å in alkenes