正在加载图片...
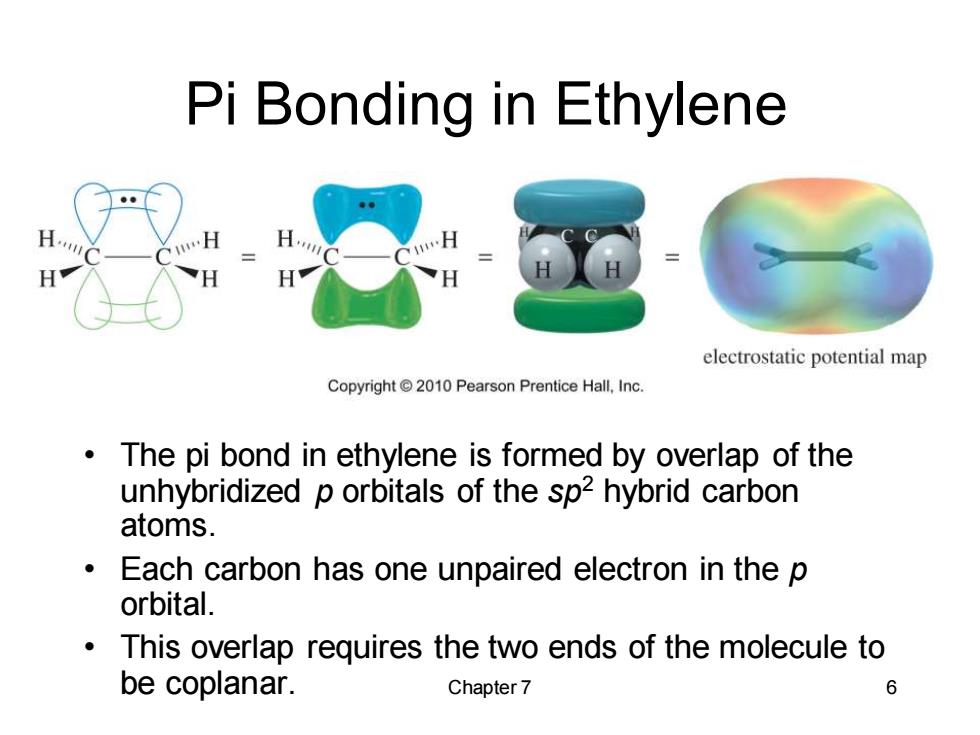
Pi Bonding in Ethylene electrostatic potential map Copyright 2010 Pearson Prentice Hall,Inc. The pi bond in ethylene is formed by overlap of the unhybridized p orbitals of the sp2 hybrid carbon atoms. Each carbon has one unpaired electron in the p orbital. This overlap requires the two ends of the molecule to be coplanar. Chapter 7 6 Chapter 7 6 Pi Bonding in Ethylene • The pi bond in ethylene is formed by overlap of the unhybridized p orbitals of the sp2 hybrid carbon atoms. • Each carbon has one unpaired electron in the p orbital. • This overlap requires the two ends of the molecule to be coplanar