正在加载图片...
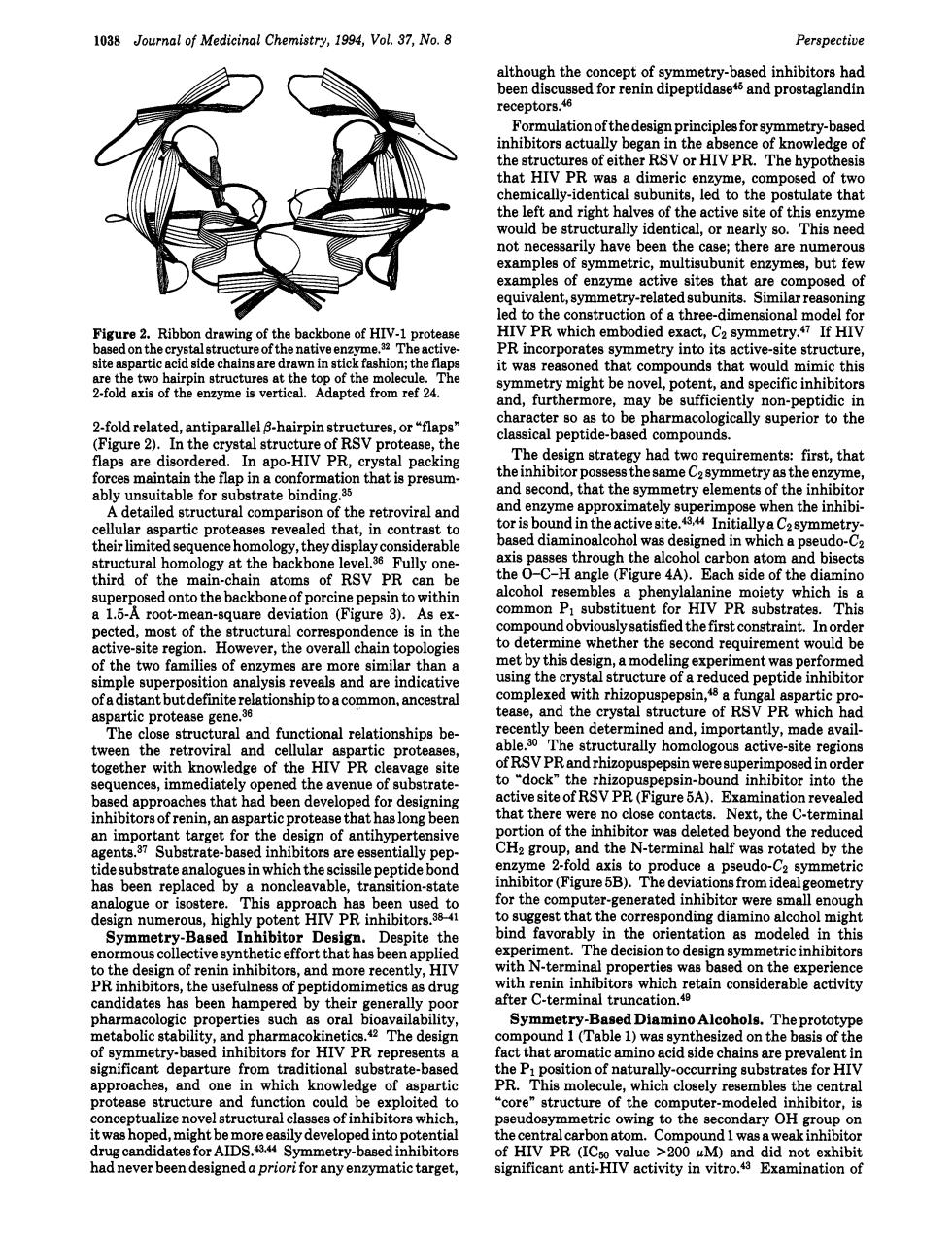
1038 Journal of Medicinal Chemistry,1994,Vol.37.No.8 Perspective multion of foymetry-b HIV PR. hat a dim ric ae. ompose of tw he left and righ of the turally identical,ore arly so. This nee les of y amples of enzyme active sites thatr compose to which ruc ofthree-dmenod spart t was reasoned chat compo ds th woul nd.furthermore. non-r 2oldrnehtehaniee aps are dis The had t firs ibit or poss metry as the e detailed structural comparison of the etroviral and tia derabl pd bise of RSV PR &"Pg26d0ntothebackbo of porcine peps ithi P subst entor rates This e cted,most of the overall chai the s ment would b ever,the by this t w lekedhhiz 36 the cryst of RSVPR which ftnctionalrelationships region edge of the HIV PR cav pin-ho nd inhibito ve site nati re c D ted b ndthe the hl tide fold erm rotated by the tor (Figure 5B).The eometr nding Desig iteth avorably 01e as mod rmi experien R ir ss of meti rope such aila it与 itors for HIV PR rep act ich l wcycgCSrtingaubtrateforH structure fun structure of the omputer -modele nhibitor t was ho might h AIDS. ly develop di, arget cant ant activity in t dnlt0not1038 Journal of Medicinal Chemistry, 1994, Vol. 37, No. 8 Perspective Figure 2. Ribbon drawing of the backbone of HIV-1 protease based on the crystal structure of the native enzyme.32 The activesite aspartic acid side chains are drawn in stick fashion; the flaps are the two hairpin structures at the top of the molecule. The 2-fold axis of the enzyme is vertical. Adapted from ref 24. 2-fold related, antiparallel 0-hairpin structures, or “flaps” (Figure 2). In the crystal structure of RSV protease, the flaps are disordered. In apo-HIV PR, crystal packing forces maintain the flap in a conformation that is presumably unsuitable for substrate binding.35 A detailed structural comparison of the retroviral and cellular aspartic proteases revealed that, in contrast to their limited sequence homology, they display considerable structural homology at the backbone le~e1.3~ Fully onethird of the main-chain atoms of RSV PR can be superposed onto the backbone of porcine pepsin to within a 1.5-A root-mean-square deviation (Figure 3). As expected, most of the structural correspondence is in the active-site region. However, the overall chain topologies of the two families of enzymes are more similar than a simple superposition analysis reveals and are indicative of a distant but definite relationship to a common, ancestral aspartic protease gene.36 The close structural and functional relationships between the retroviral and cellular aspartic proteases, together with knowledge of the HIV PR cleavage site sequences, immediately opened the avenue of substratebased approaches that had been developed for designing inhibitors of renin, an aspartic protease that has long been an important target for the design of antihypertensive agents.37 Substrate-based inhibitors are essentially peptide substrate analogues in which the scissile peptide bond has been replaced by a noncleavable, transition-state analogue or isostere. This approach has been used to design numerous, highly potent HIV PR inhibitors.3w1 Symmetry-Based Inhibitor Design. Despite the enormous collective synthetic effort that has been applied to the design of renin inhibitors, and more recently, HIV PR inhibitors, the usefulness of peptidomimetics as drug candidates has been hampered by their generally poor pharmacologic properties such as oral bioavailability, metabolic stability, and pharmacokinetics.42 The design of symmetry-based inhibitors for HIV PR represents a significant departure from traditional substrate-based approaches, and one in which knowledge of aspartic protease structure and function could be exploited to conceptualize novel structural classes of inhibitors which, it was hoped, might be more easily developed into potential drug candidates for AIDS.a*44 Symmetry-based inhibitors had never been designed a priori for any enzymatic target, although the concept of symmetry-based inhibitors had been discussed for renin dipeptidase45 and prostaglandin receptors.& Formulation of the design principles for symmetry-based inhibitors actually began in the absence of knowledge of the structures of either RSV or HIV PR. The hypothesis that HIV PR was a dimeric enzyme, composed of two chemically-identical subunits, led to the postulate that the left and right halves of the active site of this enzyme would be structurally identical, or nearly so. This need not necessarily have been the case; there are numerous examples of symmetric, multisubunit enzymes, but few examples of enzyme active sites that are composed of equivalent, symmetry-related subunits. Similar reasoning led to the construction of a three-dimensional model for HIV PR which embodied exact, CZ If HIV PR incorporates symmetry into its active-site structure, it was reasoned that compounds that would mimic this symmetry might be novel, potent, and specific inhibitors and, furthermore, may be sufficiently non-peptidic in character so as to be pharmacologically superior to the classical peptide-based compounds. The design strategy had two requirements: first, that the inhibitor possess the same CZ symmetry as the enzyme, and second, that the symmetry elements of the inhibitor and enzyme approximately superimpose when the inhibitor is bound in the active ~ite.~~~~ Initially a CZ symmetrybased diaminoalcohol was designed in which a pseudo-C2 axis passes through the alcohol carbon atom and bisects the 0-C-H angle (Figure 4A). Each side of the diamino alcohol resembles a phenylalanine moiety which is a common PI substituent for HIV PR substrates. This compound obviously satisfied the first constraint. In order to determine whether the second requirement would be met by this design, a modeling experiment was performed using the crystal structure of a reduced peptide inhibitor complexed with rhizopuspepsin,& a fungal aspartic protease, and the crystal structure of RSV PR which had recently been determined and, importantly, made available.30 The structurally homologous active-site regions of RSV PR and rhizopuspepsin were superimposed in order to “dock” the rhizopuspepsin-bound inhibitor into the active site of RSV PR (Figure 5A). Examination revealed that there were no close contacts. Next, the C-terminal portion of the inhibitor was deleted beyond the reduced CH2 group, and the N-terminal half was rotated by the enzyme 2-fold axis to produce a pseudo-C2 symmetric inhibitor (Figure 5B). The deviations from ideal geometry for the computer-generated inhibitor were small enough to suggest that the corresponding diamino alcohol might bind favorably in the orientation as modeled in this experiment. The decision to design symmetric inhibitors with N-terminal properties was based on the experience with renin inhibitors which retain considerable activity after C-terminal truncation.40 Symmetry-Based Diamino Alcohols. The prototype compound 1 (Table 1) was synthesized on the basis of the fact that aromatic amino acid side chains are prevalent in the PI position of naturally-occurring substrates for HIV PR. This molecule, which closely resembles the central %ore” structure of the computer-modeled inhibitor, is pseudosymmetric owing to the secondary OH group on the central carbon atom. Compound 1 was a weak inhibitor of HIV PR (I& value >200 pM) and did not exhibit significant anti-HIV activity in vitro.43 Examination of