正在加载图片...
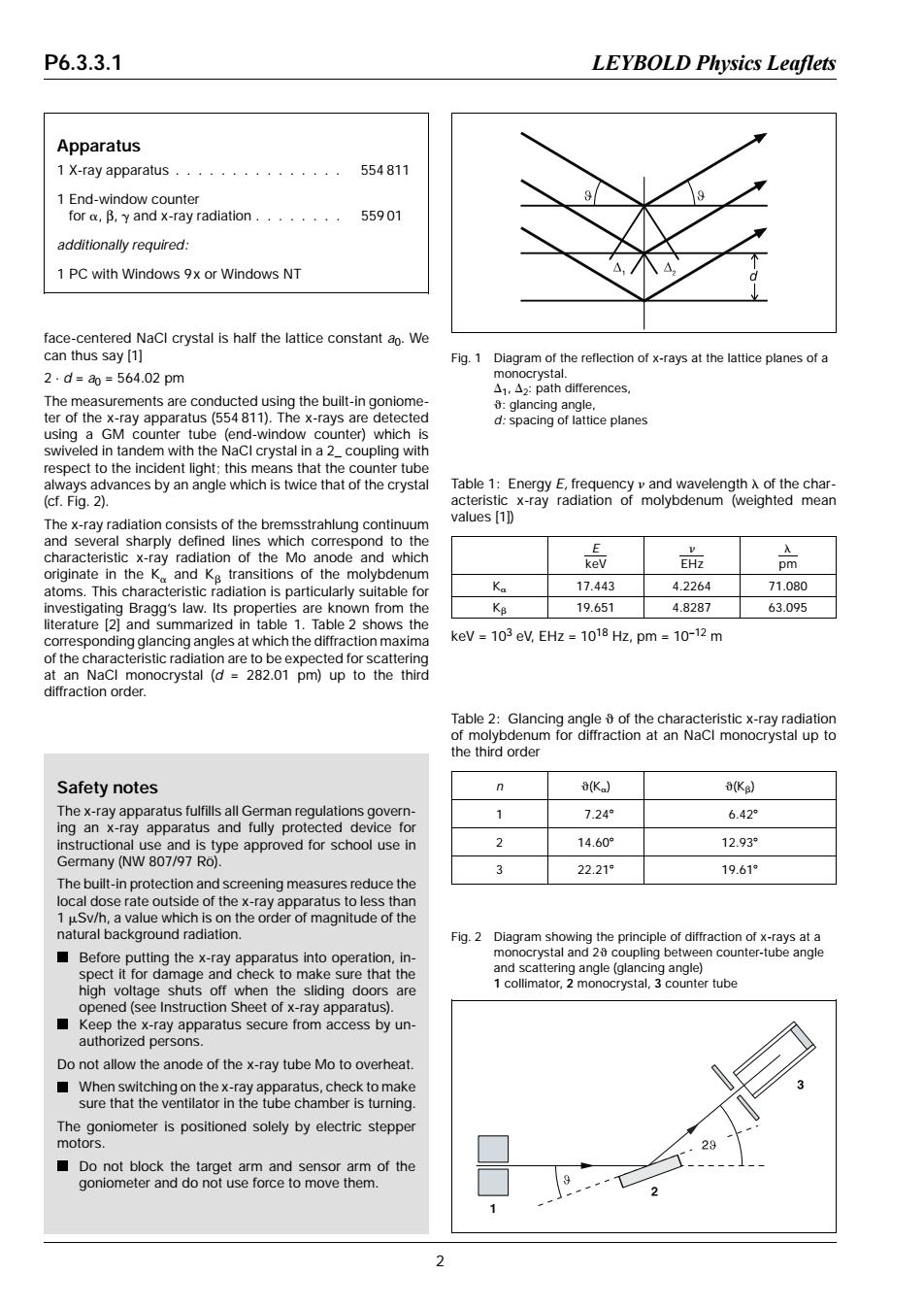
P6.3.3.1 LEYBOLD Physics Leaflets Apparatus 1X-ray apparatus.·············· 554811 1 End-window counter for,B,y and x-ray radiation.·...··· 55901 additionally required: 1 PC with Windows 9x or Windows NT face-centered NaCl crystal is half the lattice constant ao.We can thus say [1] Fig.1 Diagram of the reflection of x-rays at the lattice planes of a 2.d=a0=564.02pm monocrystal. △i,△z:path differences, The measurements are conducted using the built-in goniome- glancing angle. ter of the x-ray apparatus(554 811).The x-rays are detected d:spacing of lattice planes using a GM counter tube (end-window counter)which is swiveled in tandem with the NaCI crystal in a 2_coupling with respect to the incident light;this means that the counter tube always advances by an angle which is twice that of the crystal Table 1:Energy E,frequency v and wavelength A of the char- (cf.Fig.2). acteristic x-ray radiation of molybdenum (weighted mean The x-ray radiation consists of the bremsstrahlung continuum values [1]) and several sharply defined lines which correspond to the characteristic x-ray radiation of the Mo anode and which 总 EHz pm originate in the Ka and Kg transitions of the molybdenum atoms.This characteristic radiation is particularly suitable for Ka 17.443 4.2264 71.080 investigating Bragg's law.Its properties are known from the KB 19.651 4.8287 63.095 literature [2]and summarized in table 1.Table 2 shows the corresponding glancing angles at which the diffraction maxima keV =103 eV,EHz =1018 Hz,pm 10-12 m of the characteristic radiation are to be expected for scattering at an NaCl monocrystal (d =282.01 pm)up to the third diffraction order. Table 2:Glancing angle of the characteristic x-ray radiation of molybdenum for diffraction at an NaCl monocrystal up to the third order Safety notes 8(K (KB) The x-ray apparatus fulfills all German regulations govern- 1 7.24° 6.42 ing an x-ray apparatus and fully protected device for instructional use and is type approved for school use in 2 14.60° 12.93° Germany (NW 807/97 Ro). 3 22.21° 19.61° The built-in protection and screening measures reduce the local dose rate outside of the x-ray apparatus to less than 1 uSv/h,a value which is on the order of magnitude of the natural background radiation. Fig.2 Diagram showing the principle of diffraction of x-rays at a Before putting the x-ray apparatus into operation,in- monocrystal and 2 coupling between counter-tube angle spect it for damage and check to make sure that the and scattering angle(glancing angle) high voltage shuts off when the sliding doors are 1 collimator,2 monocrystal,3 counter tube opened(see Instruction Sheet of x-ray apparatus). Keep the x-ray apparatus secure from access by un- authorized persons. Do not allow the anode of the x-ray tube Mo to overheat When switching on the x-ray apparatus,check to make sure that the ventilator in the tube chamber is turning. The goniometer is positioned solely by electric stepper motors. Do not block the target arm and sensor arm of the goniometer and do not use force to move them. 2face-centered NaCl crystal is half the lattice constant a0. We can thus say [1] 2 ⋅ d = a0 = 564.02 pm The measurements are conducted using the built-in goniometer of the x-ray apparatus (554 811). The x-rays are detected using a GM counter tube (end-window counter) which is swiveled in tandem with the NaCl crystal in a 2_ coupling with respect to the incident light; this means that the counter tube always advances by an angle which is twice that of the crystal (cf. Fig. 2). The x-ray radiation consists of the bremsstrahlung continuum and several sharply defined lines which correspond to the characteristic x-ray radiation of the Mo anode and which originate in the Ka and Kb transitions of the molybdenum atoms. This characteristic radiation is particularly suitable for investigating Bragg’s law. Its properties are known from the literature [2] and summarized in table 1. Table 2 shows the corresponding glancing angles at which the diffraction maxima of the characteristic radiation are to be expected for scattering at an NaCl monocrystal (d = 282.01 pm) up to the third diffraction order. E keV n EHz l pm Ka 17.443 4.2264 71.080 Kb 19.651 4.8287 63.095 keV = 103 eV, EHz = 1018 Hz, pm = 10–12 m Table 1: Energy E, frequency n and wavelength l of the characteristic x-ray radiation of molybdenum (weighted mean values [1]) n q(Ka) q(Kb) 1 7.248 6.428 2 14.608 12.938 3 22.218 19.618 Table 2: Glancing angle q of the characteristic x-ray radiation of molybdenum for diffraction at an NaCl monocrystal up to the third order Apparatus 1 X-ray apparatus . . . . . . . . . . . . . . . 554 811 1 End-window counter for a, β, g and x-ray radiation . . . . . . . . 559 01 additionally required: 1 PC with Windows 9x or Windows NT Safety notes The x-ray apparatus fulfills all German regulations governing an x-ray apparatus and fully protected device for instructional use and is type approved for school use in Germany (NW 807/97 Rö). The built-in protection and screening measures reduce the local dose rate outside of the x-ray apparatus to less than 1 mSv/h, a value which is on the order of magnitude of the natural background radiation. Before putting the x-ray apparatus into operation, inspect it for damage and check to make sure that the high voltage shuts off when the sliding doors are opened (see Instruction Sheet of x-ray apparatus). Keep the x-ray apparatus secure from access by unauthorized persons. Do not allow the anode of the x-ray tube Mo to overheat. When switching on the x-ray apparatus, check to make sure that the ventilator in the tube chamber is turning. The goniometer is positioned solely by electric stepper motors. Do not block the target arm and sensor arm of the goniometer and do not use force to move them. Fig. 2 Diagram showing the principle of diffraction of x-rays at a monocrystal and 2q coupling between counter-tube angle and scattering angle (glancing angle) 1 collimator, 2 monocrystal, 3 counter tube Fig. 1 Diagram of the reflection of x-rays at the lattice planes of a monocrystal. D1, D2: path differences, q: glancing angle, d: spacing of lattice planes P6.3.3.1 LEYBOLD Physics Leaflets 2