正在加载图片...
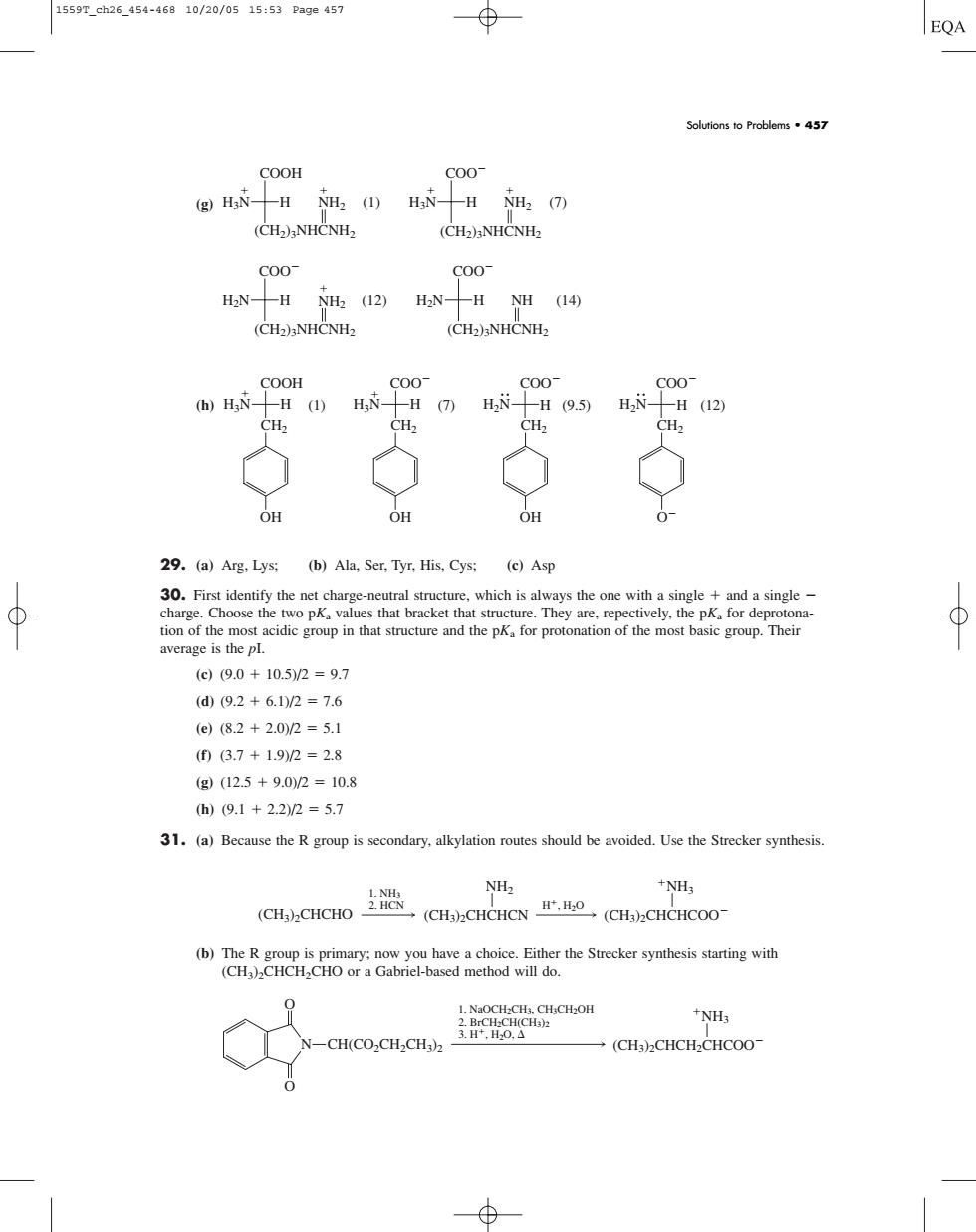
1559T_ch26_454-46810/20/0515:53Pa9e457 EQA Solutionsto Problems457 COOH 900 (g)HN-H NH2 (1)HN-H NH2 (7) (CH)NHCNH> (CH2)3NHCNHz C00 C00 HaN--H NH2 (12) H2N-H NH (14) (CH2)NHCNHz 900 H (1)H. H (7)H2 H(9.5)H, H(12) 12 CH OH OH 0- 29.(a)Arg.Lys:(b)Ala.Ser.Tyr.His,Cys:(e)Asp single and a single roup.Their average is the pl. ()(9.0+10.5/2=9.7 (d(9.2+6.1)/2=7.6 (e(8.2+2.0/2=5.1 (0(6.7+1.92=2.8 (g(12.5+9.0M2=10.8 h)(9.1+2.2)/2=5.7 31.(a)Because the R group is secondary.alkylation routes should be avoided.Use the Strecker synthesis. NH3 CH(CO,CH,CH) (g) (h) 29. (a) Arg, Lys; (b) Ala, Ser, Tyr, His, Cys; (c) Asp 30. First identify the net charge-neutral structure, which is always the one with a single and a single charge. Choose the two pKa values that bracket that structure. They are, repectively, the pKa for deprotonation of the most acidic group in that structure and the pKa for protonation of the most basic group. Their average is the pI. (c) (9.0 10.5)/2 9.7 (d) (9.2 6.1)/2 7.6 (e) (8.2 2.0)/2 5.1 (f) (3.7 1.9)/2 2.8 (g) (12.5 9.0)/2 10.8 (h) (9.1 2.2)/2 5.7 31. (a) Because the R group is secondary, alkylation routes should be avoided. Use the Strecker synthesis. (b) The R group is primary; now you have a choice. Either the Strecker synthesis starting with (CH3)2CHCH2CHO or a Gabriel-based method will do. 1. NaOCH2CH3, CH3CH2OH 2. BrCH2CH(CH3)2 3. H, H2O, NH3 N O O CH(CO2CH2CH3)2 (CH3)2CHCH2CHCOO H, H2O 1. NH3 2. HCN (CH3)2CHCHO (CH3)2CHCHCN NH2 (CH3)2CHCHCOO NH3 (1) COO COO COO O H3N H COOH CH2 OH H3N (7) H CH2 OH H2N H (9.5) CH2 OH H2N H (12) CH2 H3N NH2 (1) H COOH (CH2)3NHCNH2 H3N (7) H COO H2N H (12) COO H2N H (14) COO NH2 (CH2)3NHCNH2 NH2 (CH2)3NHCNH2 NH (CH2)3NHCNH2 Solutions to Problems • 457 1559T_ch26_454-468 10/20/05 15:53 Page 457����������������