正在加载图片...
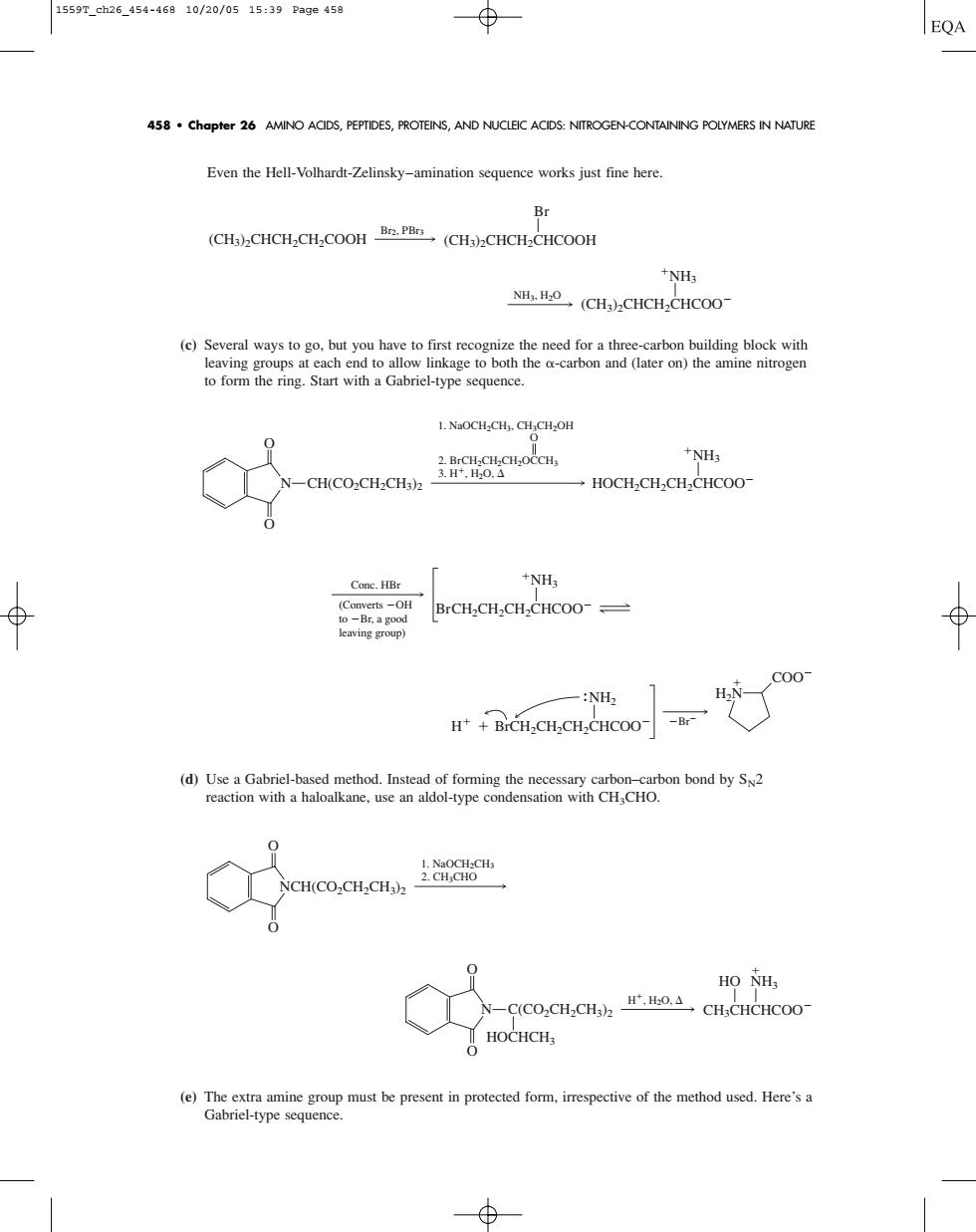
1559r.eh26.454-46810/20/0515:39Page458 458 chapter 26 AMINO ACIDS,PEPTIDES,PROTEINS,AND NUCLEK ACIDS:NITROGEN-CONTAINING POLYMERS IN NATURE Even the Hell-Volhardt-Zelinskyamination sequence works just fine here Br CH)CHCHCH-COOH(CH)CHCH-CHCOOH NHs (CH-CHCH CHCOO- (c)Several ways to go,but you have to first recognize the need for a three-carbon building block with leaving groups at 1.NaOCH.CH.CH.CH.OH -CH(COCH-CH COC.OCCH. NH3 Cone.HBr NH; BrCH,CH2CH-CHCOO- aving group raaawr"y 一N NCHCO.CH.CH HO NHs C(CO,CH,CH).H. CH:CHCHCOO- HOCHCH (e)The extra amine group must be present in protected form.irrespective of the method used.Here'sa Gabriel-type sequencEven the Hell-Volhardt-Zelinsky–amination sequence works just fine here. (c) Several ways to go, but you have to first recognize the need for a three-carbon building block with leaving groups at each end to allow linkage to both the -carbon and (later on) the amine nitrogen to form the ring. Start with a Gabriel-type sequence. (d) Use a Gabriel-based method. Instead of forming the necessary carbon–carbon bond by SN2 reaction with a haloalkane, use an aldol-type condensation with CH3CHO. (e) The extra amine group must be present in protected form, irrespective of the method used. Here’s a Gabriel-type sequence. H, H2O, NH3 HO CH3CHCHCOO N O O C(CO2CH2CH3)2 HOCHCH3 1. NaOCH2CH3 2. CH3CHO O O NCH(CO2CH2CH3)2 NH2 BrCH2CH2CH2CHCOO COO Br H2N H Conc. HBr (Converts OH to Br, a good leaving group) NH3 BrCH2CH2CH2CHCOO 1. NaOCH2CH3, CH3CH2OH 2. BrCH2CH2CH2OCCH3 O 3. H, H2O, N O O CH(CO2CH2CH3)2 NH3 HOCH2CH2CH2CHCOO NH3, H2O (CH3)2CHCH2CHCOO NH3 Br2, PBr3 (CH3)2CHCH2CH2COOH Br (CH3)2CHCH2CHCOOH 458 • Chapter 26 AMINO ACIDS, PEPTIDES, PROTEINS, AND NUCLEIC ACIDS: NITROGEN-CONTAINING POLYMERS IN NATURE 1559T_ch26_454-468 10/20/05 15:39 Page 458�������������������