正在加载图片...
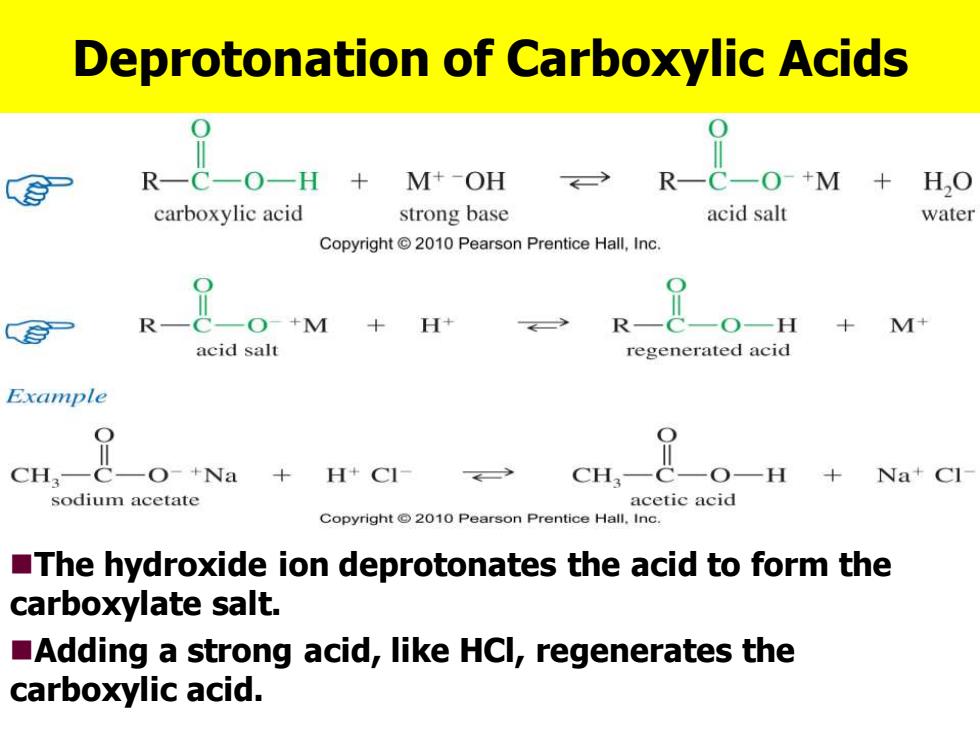
Deprotonation of Carboxylic Acids R-C-O-H M+-OH R-C一O+M HO carboxylic acid strong base acid salt water Copyright2010 Pearson Prentice Hall,Inc. R-C一O+M+H R-C-O-H +M+ acid salt regenerated acid Example CH.-c-o-+Na +H+CI- cH,-&-0-H +Na+Cl sodium acetate acetic acid Copyright2010 Pearson Prentice Hall.Inc. The hydroxide ion deprotonates the acid to form the carboxylate salt. Adding a strong acid,like HCl,regenerates the carboxylic acid. Deprotonation of Carboxylic Acids ◼The hydroxide ion deprotonates the acid to form the carboxylate salt. ◼Adding a strong acid, like HCl, regenerates the carboxylic acid