正在加载图片...
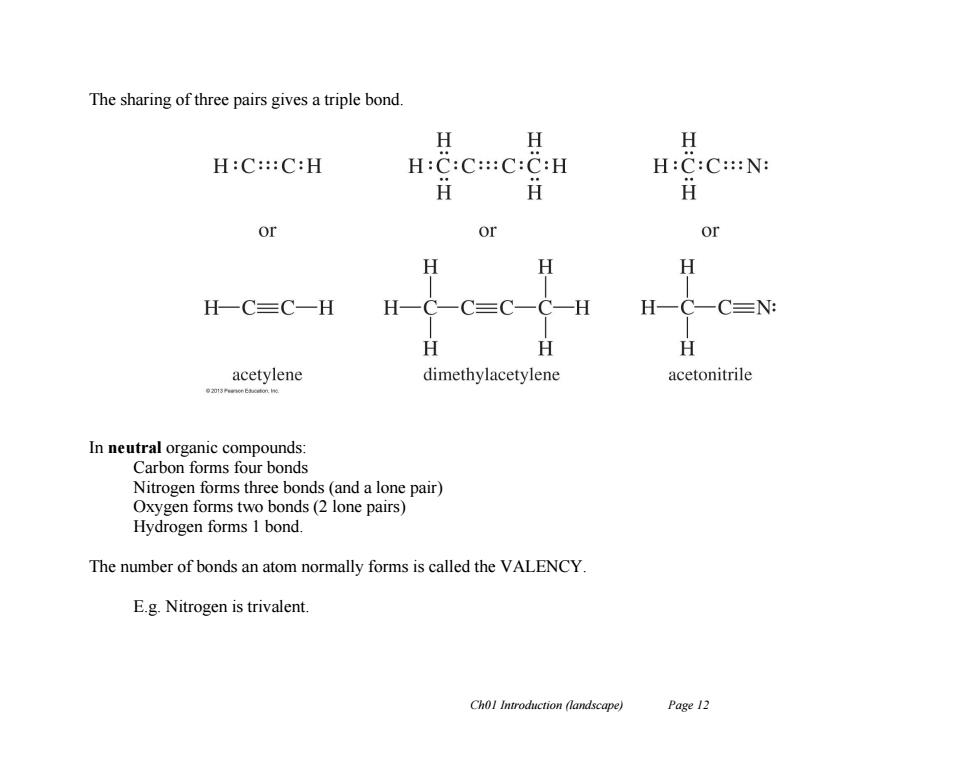
The sharing of three pairs gives a triple bond. H 女 H H:C:::C:H H:C:C:::C:C:H H:C:C::N: H or or or H H H H一C三C一H H -H H-C- CN: H H H acetylene dimethylacetylene acetonitrile In neutral organic compounds: Carbon forms four bonds Nitrogen forms three bonds(and a lone pair) Oxygen forms two bonds(2 lone pairs) Hydrogen forms 1 bond. The number of bonds an atom normally forms is called the VALENCY. E.g.Nitrogen is trivalent. Chol Introduction (landscape) Page 12Ch01 Introduction (landscape) Page 12 The sharing of three pairs gives a triple bond. In neutral organic compounds: Carbon forms four bonds Nitrogen forms three bonds (and a lone pair) Oxygen forms two bonds (2 lone pairs) Hydrogen forms 1 bond. The number of bonds an atom normally forms is called the VALENCY. E.g. Nitrogen is trivalent