正在加载图片...
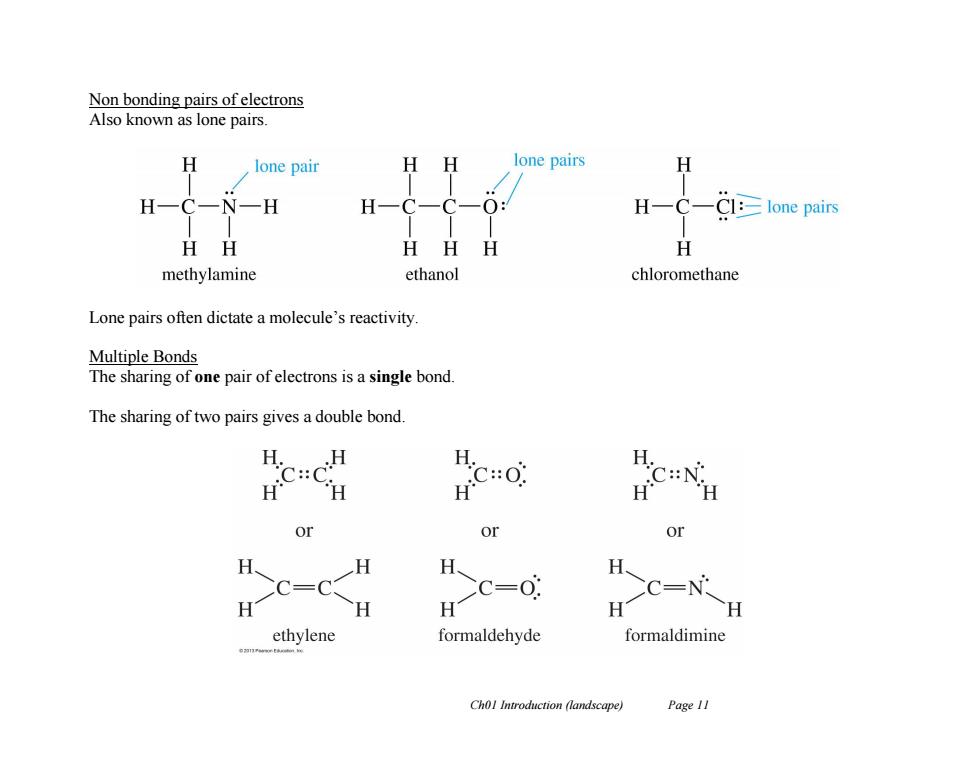
Non bonding pairs of electrons Also known as lone pairs. lone pair H lone pairs H H N一H H一C一 O: HC—Cl:≥lone pairs HH HHH 任 methylamine ethanol chloromethane Lone pairs often dictate a molecule's reactivity Multiple Bonds The sharing of one pair of electrons is a single bond. The sharing of two pairs gives a double bond. H.H H. C::C H.C:0 C:N: or or or H、 H H、 H CC=C C=0 CC=N H H H H H ethylene formaldehyde formaldimine Chol Introduction (landscape) Page 11 Ch01 Introduction (landscape) Page 11 Non bonding pairs of electrons Also known as lone pairs. Lone pairs often dictate a molecule’s reactivity. Multiple Bonds The sharing of one pair of electrons is a single bond. The sharing of two pairs gives a double bond