正在加载图片...
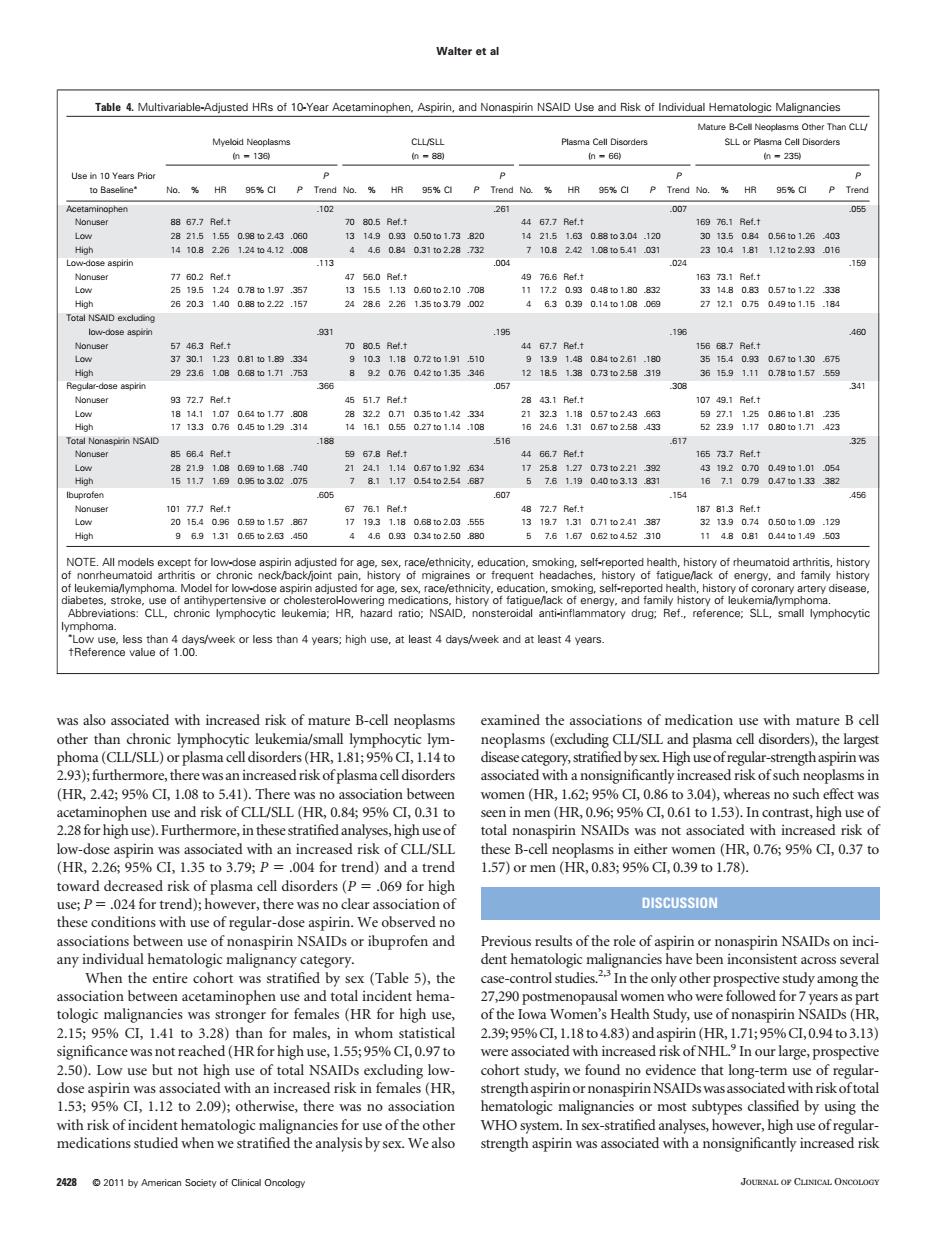
Walter ta Table4.Mutivariable-Adusted HRs of 10-Year Acetaminophen,Aspirn,and Nonan NSAID Use and Risk ot Individual Hemaoogc Maignancies 的23 Nonus 7 0.2 Ret.t 49 766 Ref.t 的 195 Nonu OTE.All models except for low Roreehan10ereMekoclesthan4yearg;hghuse,arleast4dayarekandatleasr4years ma(CIL/SLL)or plasma celldisorders (HR.1%Cl1.14to stratified bysex High uscofregular-st gth aspirin wa aociatedwhanonsignihcantiyincreasedrhkofsuchneoplhasmsi 00fCSR08495%C.031i total nonaspirin NSAIDs was not associated with increased riskof toward decreased risk of plasma cell disorders(P=069 for high c..0 or mer DISCUSSION When the entire cohort was stratified by sex (Table 5),the 2.1595%C,1.41to3.28)tha 2.3995%C,1.18to4.83)and aspirin(HR1.71:95%C,0.94to3.13 d(HRf 5595% ated v 1.53%Cl,1.12 to 2.09);otherwise,there was no association natologic malignancies or most subtypes classified by using the was also associated with increased risk of mature B-cell neoplasms other than chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) or plasma cell disorders (HR, 1.81; 95% CI, 1.14 to 2.93);furthermore, therewas anincreased risk of plasma cell disorders (HR, 2.42; 95% CI, 1.08 to 5.41). There was no association between acetaminophen use and risk of CLL/SLL (HR, 0.84; 95% CI, 0.31 to 2.28for high use). Furthermore,in these stratified analyses, high use of low-dose aspirin was associated with an increased risk of CLL/SLL (HR, 2.26; 95% CI, 1.35 to 3.79; P .004 for trend) and a trend toward decreased risk of plasma cell disorders (P .069 for high use; P .024 for trend); however, there was no clear association of these conditions with use of regular-dose aspirin. We observed no associations between use of nonaspirin NSAIDs or ibuprofen and any individual hematologic malignancy category. When the entire cohort was stratified by sex (Table 5), the association between acetaminophen use and total incident hematologic malignancies was stronger for females (HR for high use, 2.15; 95% CI, 1.41 to 3.28) than for males, in whom statistical significance was not reached (HR for high use, 1.55; 95% CI, 0.97 to 2.50). Low use but not high use of total NSAIDs excluding lowdose aspirin was associated with an increased risk in females (HR, 1.53; 95% CI, 1.12 to 2.09); otherwise, there was no association with risk of incident hematologic malignancies for use of the other medications studied when we stratified the analysis by sex. We also examined the associations of medication use with mature B cell neoplasms (excluding CLL/SLL and plasma cell disorders), the largest disease category, stratified by sex.High use of regular-strength aspirinwas associated with a nonsignificantly increased risk of such neoplasms in women (HR, 1.62; 95% CI, 0.86 to 3.04), whereas no such effect was seen in men (HR, 0.96; 95% CI, 0.61 to 1.53). In contrast, high use of total nonaspirin NSAIDs was not associated with increased risk of these B-cell neoplasms in either women (HR, 0.76; 95% CI, 0.37 to 1.57) or men (HR, 0.83; 95% CI, 0.39 to 1.78). DISCUSSION Previous results of the role of aspirin or nonaspirin NSAIDs on incident hematologic malignancies have been inconsistent across several case-control studies.2,3 In the only other prospective study among the 27,290 postmenopausal women who were followed for 7 years as part of the Iowa Women’s Health Study, use of nonaspirin NSAIDs (HR, 2.39; 95%CI, 1.18 to 4.83) and aspirin (HR, 1.71; 95%CI, 0.94 to 3.13) were associated with increased risk of NHL.9 In our large, prospective cohort study, we found no evidence that long-term use of regularstrength aspirin or nonaspirinNSAIDswas associatedwith risk of total hematologic malignancies or most subtypes classified by using the WHO system. In sex-stratified analyses, however, high use of regularstrength aspirin was associated with a nonsignificantly increased risk Table 4. Multivariable-Adjusted HRs of 10-Year Acetaminophen, Aspirin, and Nonaspirin NSAID Use and Risk of Individual Hematologic Malignancies Use in 10 Years Prior to Baseline Myeloid Neoplasms (n 136) CLL/SLL (n 88) Plasma Cell Disorders (n 66) Mature B-Cell Neoplasms Other Than CLL/ SLL or Plasma Cell Disorders (n 235) No. % HR 95% CI P P Trend No. % HR 95% CI P P Trend No. % HR 95% CI P P Trend No. % HR 95% CI P P Trend Acetaminophen .102 .261 .007 .055 Nonuser 88 67.7 Ref.† 70 80.5 Ref.† 44 67.7 Ref.† 169 76.1 Ref.† Low 28 21.5 1.55 0.98 to 2.43 .060 13 14.9 0.93 0.50 to 1.73 .820 14 21.5 1.63 0.88 to 3.04 .120 30 13.5 0.84 0.56 to 1.26 .403 High 14 10.8 2.26 1.24 to 4.12 .008 4 4.6 0.84 0.31 to 2.28 .732 7 10.8 2.42 1.08 to 5.41 .031 23 10.4 1.81 1.12 to 2.93 .016 Low-dose aspirin .113 .004 .024 .159 Nonuser 77 60.2 Ref.† 47 56.0 Ref.† 49 76.6 Ref.† 163 73.1 Ref.† Low 25 19.5 1.24 0.78 to 1.97 .357 13 15.5 1.13 0.60 to 2.10 .708 11 17.2 0.93 0.48 to 1.80 .832 33 14.8 0.83 0.57 to 1.22 .338 High 26 20.3 1.40 0.88 to 2.22 .157 24 28.6 2.26 1.35 to 3.79 .002 4 6.3 0.39 0.14 to 1.08 .069 27 12.1 0.75 0.49 to 1.15 .184 Total NSAID excluding low-dose aspirin .931 .195 .196 .460 Nonuser 57 46.3 Ref.† 70 80.5 Ref.† 44 67.7 Ref.† 156 68.7 Ref.† Low 37 30.1 1.23 0.81 to 1.89 .334 9 10.3 1.18 0.72 to 1.91 .510 9 13.9 1.48 0.84 to 2.61 .180 35 15.4 0.93 0.67 to 1.30 .675 High 29 23.6 1.08 0.68 to 1.71 .753 8 9.2 0.76 0.42 to 1.35 .346 12 18.5 1.38 0.73 to 2.58 .319 36 15.9 1.11 0.78 to 1.57 .559 Regular-dose aspirin .366 .057 .308 .341 Nonuser 93 72.7 Ref.† 45 51.7 Ref.† 28 43.1 Ref.† 107 49.1 Ref.† Low 18 14.1 1.07 0.64 to 1.77 .808 28 32.2 0.71 0.35 to 1.42 .334 21 32.3 1.18 0.57 to 2.43 .663 59 27.1 1.25 0.86 to 1.81 .235 High 17 13.3 0.76 0.45 to 1.29 .314 14 16.1 0.55 0.27 to 1.14 .108 16 24.6 1.31 0.67 to 2.58 .433 52 23.9 1.17 0.80 to 1.71 .423 Total Nonaspirin NSAID .188 .516 .617 .325 Nonuser 85 66.4 Ref.† 59 67.8 Ref.† 44 66.7 Ref.† 165 73.7 Ref.† Low 28 21.9 1.08 0.69 to 1.68 .740 21 24.1 1.14 0.67 to 1.92 .634 17 25.8 1.27 0.73 to 2.21 .392 43 19.2 0.70 0.49 to 1.01 .054 High 15 11.7 1.69 0.95 to 3.02 .075 7 8.1 1.17 0.54 to 2.54 .687 5 7.6 1.19 0.40 to 3.13 .831 16 7.1 0.79 0.47 to 1.33 .382 Ibuprofen .605 .607 .154 .456 Nonuser 101 77.7 Ref.† 67 76.1 Ref.† 48 72.7 Ref.† 187 81.3 Ref.† Low 20 15.4 0.96 0.59 to 1.57 .867 17 19.3 1.18 0.68 to 2.03 .555 13 19.7 1.31 0.71 to 2.41 .387 32 13.9 0.74 0.50 to 1.09 .129 High 9 6.9 1.31 0.65 to 2.63 .450 4 4.6 0.93 0.34 to 2.50 .880 5 7.6 1.67 0.62 to 4.52 .310 11 4.8 0.81 0.44 to 1.49 .503 NOTE. All models except for low-dose aspirin adjusted for age, sex, race/ethnicity, education, smoking, self-reported health, history of rheumatoid arthritis, history of nonrheumatoid arthritis or chronic neck/back/joint pain, history of migraines or frequent headaches, history of fatigue/lack of energy, and family history of leukemia/lymphoma. Model for low-dose aspirin adjusted for age, sex, race/ethnicity, education, smoking, self-reported health, history of coronary artery disease, diabetes, stroke, use of antihypertensive or cholesterol-lowering medications, history of fatigue/lack of energy, and family history of leukemia/lymphoma. Abbreviations: CLL, chronic lymphocytic leukemia; HR, hazard ratio; NSAID, nonsteroidal anti-inflammatory drug; Ref., reference; SLL, small lymphocytic lymphoma. Low use, less than 4 days/week or less than 4 years; high use, at least 4 days/week and at least 4 years. †Reference value of 1.00. Walter et al 2428 © 2011 by American Society of Clinical Oncology JOURNAL OF CLINICAL ONCOLOGY