正在加载图片...
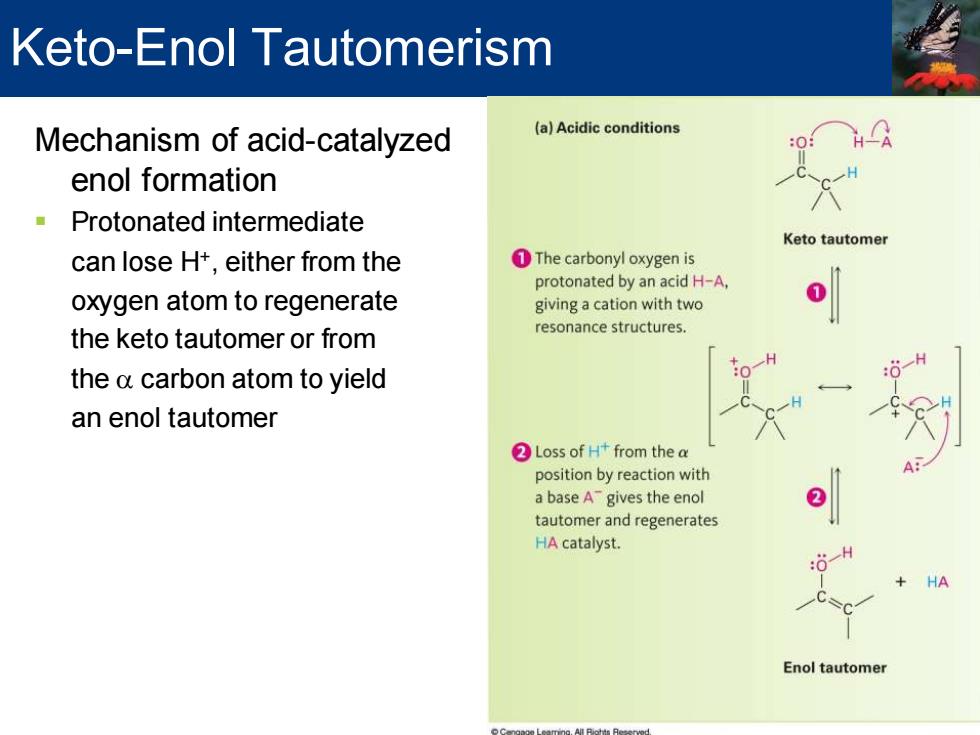
Keto-Enol Tautomerism Mechanism of acid-catalyzed (a)Acidic conditions enol formation -Protonated intermediate Keto tautomer can lose H+,either from the 1The carbonyl oxygen is protonated by an acid H-A, oxygen atom to regenerate giving a cation with two the keto tautomer or from resonance structures. the a carbon atom to yield an enol tautomer ②Loss of H+from the a position by reaction with A日 a base A gives the enol tautomer and regenerates HA catalyst. HA Enol tautomer Mechanism of acid-catalyzed enol formation ▪ Protonated intermediate can lose H+ , either from the oxygen atom to regenerate the keto tautomer or from the a carbon atom to yield an enol tautomer Keto-Enol Tautomerism