正在加载图片...
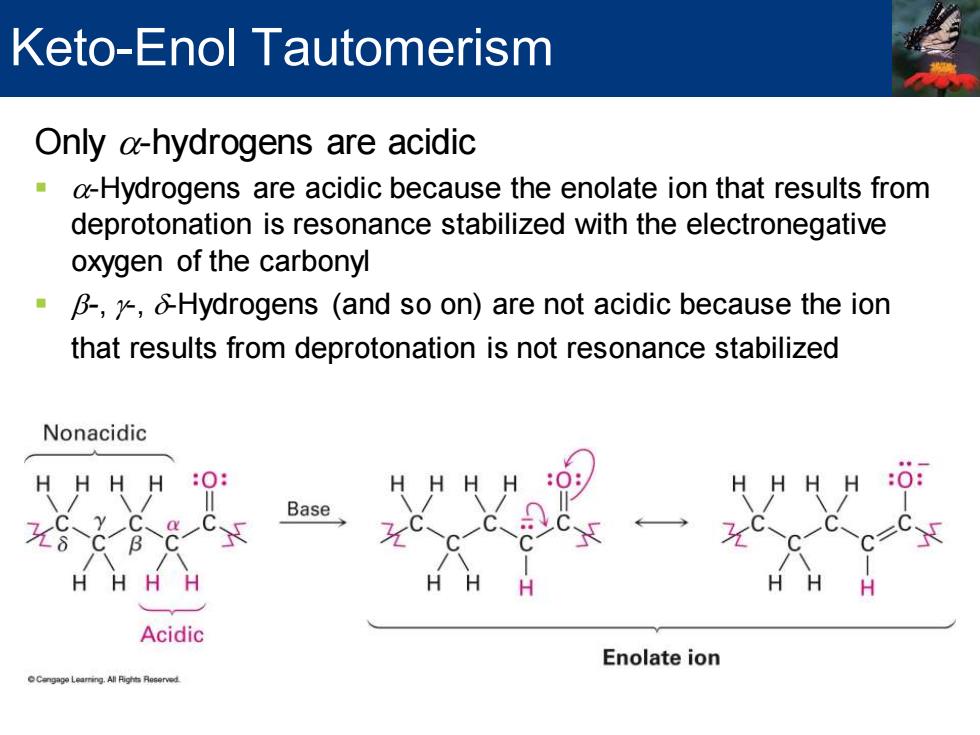
Keto-Enol Tautomerism Only a-hydrogens are acidic a-Hydrogens are acidic because the enolate ion that results from deprotonation is resonance stabilized with the electronegative oxygen of the carbonyl B-,/Hydrogens (and so on)are not acidic because the ion that results from deprotonation is not resonance stabilized Nonacidic H HH HH H :0: Base y C H HH H H H H Acidic Enolate ionOnly a-hydrogens are acidic ▪ a-Hydrogens are acidic because the enolate ion that results from deprotonation is resonance stabilized with the electronegative oxygen of the carbonyl ▪ b-, g-, d-Hydrogens (and so on) are not acidic because the ion that results from deprotonation is not resonance stabilized Keto-Enol Tautomerism