正在加载图片...
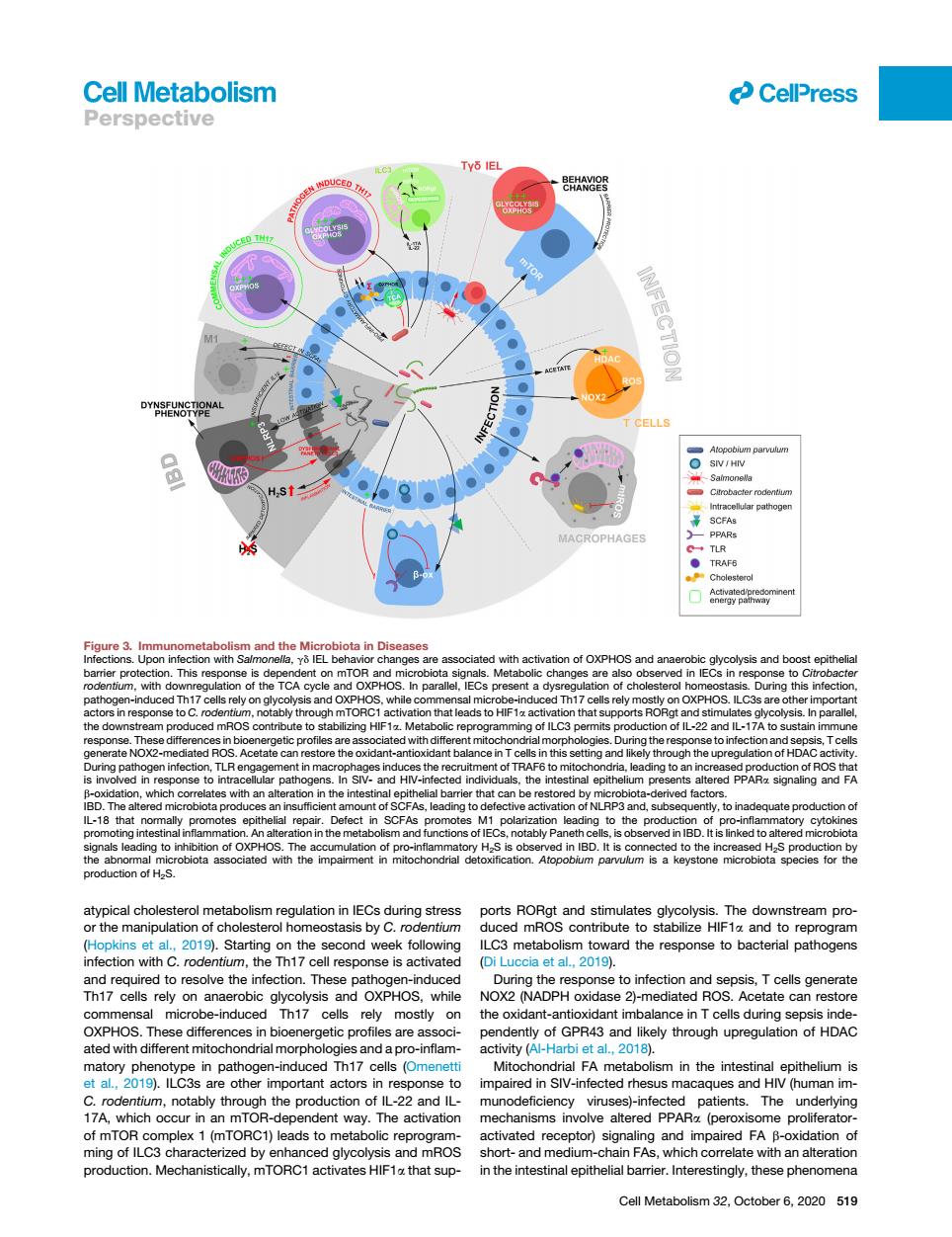
Cell Metabolism CelPress Perspective TY6 IE ● PHAGE is obse 9).Starting on econd weel to infection and sepsis,Tcellsg erate on e can restor e diffe bioen pendently of GPR43 and likely through upregulation of HDAC ated wit L,2019.c8 sm in the intest ithelium e othe important ac mpaired in SIV-infected rhesus mac es and HI human im r;I ZA which r in The 22 patients.The mplex 1 (mTORC1)leads to meta activated r tor)signaling and impa red FA Cell Metabolism 32.October 6,2020 519 atypical cholesterol metabolism regulation in IECs during stress or the manipulation of cholesterol homeostasis by C. rodentium (Hopkins et al., 2019). Starting on the second week following infection with C. rodentium, the Th17 cell response is activated and required to resolve the infection. These pathogen-induced Th17 cells rely on anaerobic glycolysis and OXPHOS, while commensal microbe-induced Th17 cells rely mostly on OXPHOS. These differences in bioenergetic profiles are associated with different mitochondrial morphologies and a pro-inflammatory phenotype in pathogen-induced Th17 cells (Omenetti et al., 2019). ILC3s are other important actors in response to C. rodentium, notably through the production of IL-22 and IL- 17A, which occur in an mTOR-dependent way. The activation of mTOR complex 1 (mTORC1) leads to metabolic reprogramming of ILC3 characterized by enhanced glycolysis and mROS production. Mechanistically, mTORC1 activates HIF1a that supports RORgt and stimulates glycolysis. The downstream produced mROS contribute to stabilize HIF1a and to reprogram ILC3 metabolism toward the response to bacterial pathogens (Di Luccia et al., 2019). During the response to infection and sepsis, T cells generate NOX2 (NADPH oxidase 2)-mediated ROS. Acetate can restore the oxidant-antioxidant imbalance in T cells during sepsis independently of GPR43 and likely through upregulation of HDAC activity (Al-Harbi et al., 2018). Mitochondrial FA metabolism in the intestinal epithelium is impaired in SIV-infected rhesus macaques and HIV (human immunodeficiency viruses)-infected patients. The underlying mechanisms involve altered PPARa (peroxisome proliferatoractivated receptor) signaling and impaired FA b-oxidation of short- and medium-chain FAs, which correlate with an alteration in the intestinal epithelial barrier. Interestingly, these phenomena Figure 3. Immunometabolism and the Microbiota in Diseases Infections. Upon infection with Salmonella, gd IEL behavior changes are associated with activation of OXPHOS and anaerobic glycolysis and boost epithelial barrier protection. This response is dependent on mTOR and microbiota signals. Metabolic changes are also observed in IECs in response to Citrobacter rodentium, with downregulation of the TCA cycle and OXPHOS. In parallel, IECs present a dysregulation of cholesterol homeostasis. During this infection, pathogen-induced Th17 cells rely on glycolysis and OXPHOS, while commensal microbe-induced Th17 cells rely mostly on OXPHOS. ILC3s are other important actors in response to C. rodentium, notably through mTORC1 activation that leads to HIF1a activation that supports RORgt and stimulates glycolysis. In parallel, the downstream produced mROS contribute to stabilizing HIF1a. Metabolic reprogramming of ILC3 permits production of IL-22 and IL-17A to sustain immune response. These differences in bioenergetic profiles are associated with different mitochondrial morphologies. During the response to infection and sepsis, T cells generate NOX2-mediated ROS. Acetate can restore the oxidant-antioxidant balance in T cells in this setting and likely through the upregulation of HDAC activity. During pathogen infection, TLR engagement in macrophages induces the recruitment of TRAF6 to mitochondria, leading to an increased production of ROS that is involved in response to intracellular pathogens. In SIV- and HIV-infected individuals, the intestinal epithelium presents altered PPARa signaling and FA b-oxidation, which correlates with an alteration in the intestinal epithelial barrier that can be restored by microbiota-derived factors. IBD. The altered microbiota produces an insufficient amount of SCFAs, leading to defective activation of NLRP3 and, subsequently, to inadequate production of IL-18 that normally promotes epithelial repair. Defect in SCFAs promotes M1 polarization leading to the production of pro-inflammatory cytokines promoting intestinal inflammation. An alteration in the metabolism and functions of IECs, notably Paneth cells, is observed in IBD. It is linked to altered microbiota signals leading to inhibition of OXPHOS. The accumulation of pro-inflammatory H2S is observed in IBD. It is connected to the increased H2S production by the abnormal microbiota associated with the impairment in mitochondrial detoxification. Atopobium parvulum is a keystone microbiota species for the production of H2S. ll Cell Metabolism 32, October 6, 2020 519 Perspective