正在加载图片...
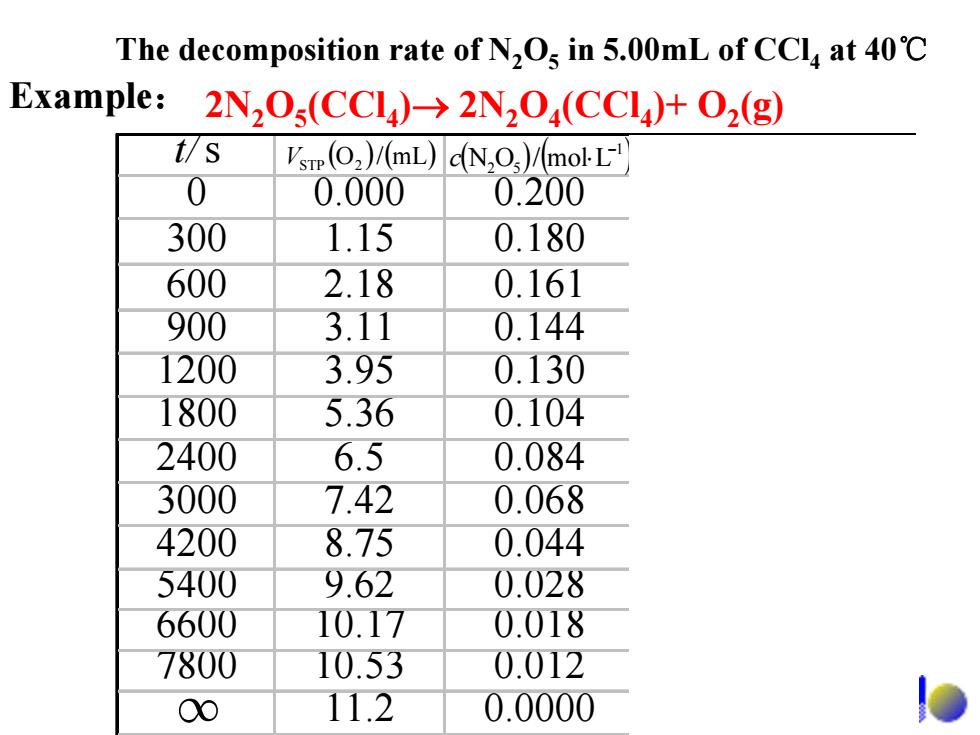
The decomposition rate of N,Os in 5.00mL of CCla at 40C Example:2N,O(CCI)>2N2O(CCI)+O2(g) t/S Vsrp(O2)/(mL)dN,O)/molL 0 0.000 0.200 300 1.15 0.180 600 2.18 0.161 900 3.11 0.144 1200 3.95 0.130 1800 5.36 0.104 2400 6.5 0.084 3000 7.42 0.068 4200 8.75 0.044 5400 9.62 0.028 6600 10.17 0.018 7800 10.53 0.012 00 11.2 0.0000t/s 0 0.000 0.200 300 1.15 0.180 600 2.18 0.161 900 3.11 0.144 1200 3.95 0.130 1800 5.36 0.104 2400 6.5 0.084 3000 7.42 0.068 4200 8.75 0.044 5400 9.62 0.028 6600 10.17 0.018 7800 10.53 0.012 11.2 0.0000 ( ) (mL/O ) V 2STP ( ) ( )1 52 Lmol/ON − c ⋅ ( )11 sLmol/ −− υ ⋅⋅ 5 1029.7 − × 5 1046.6 − × 5 1080.5 − × 5 1021.5 − × 5 1069.4 − × 5 1079.3 − × 5 1004.3 − × 5 1003.1 − × 5 1044.2 − × 5 1059.1 − × ∞ The decomposition rate of N2O5 in 5.00mL of CCl4 at 40℃ 2N2O5(CCl4)→ 2N2O4(CCl4)+ O2 Example: (g)