正在加载图片...
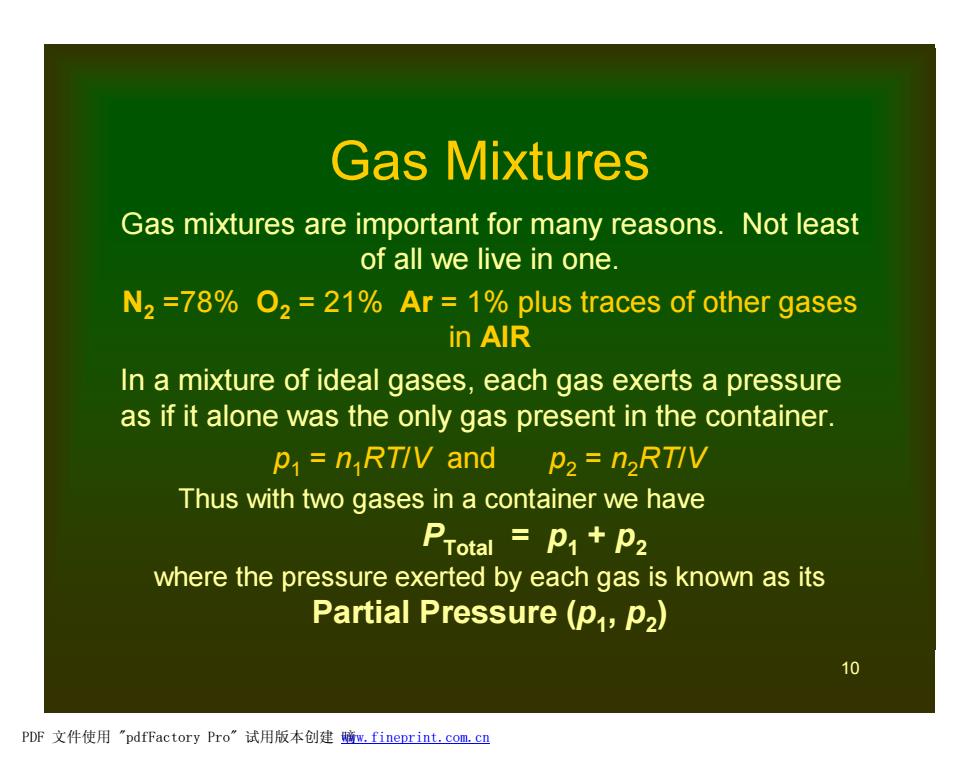
Gas Mixtures Gas mixtures are important for many reasons.Not least of all we live in one. N2=78%O2=21%Ar =1%plus traces of other gases in AlR In a mixture of ideal gases,each gas exerts a pressure as if it alone was the only gas present in the container. p=nRTIV and p2=n2RTIV Thus with two gases in a container we have PTotal p1+p2 where the pressure exerted by each gas is known as its Partial Pressure (p,P2) 10 PDF文件使用"pdfFactory Pro”试用版本创建鳍m,fineprint.com,cn10 Gas Mixtures Gas mixtures are important for many reasons. Not least of all we live in one. N2 =78% O2 = 21% Ar = 1% plus traces of other gases in AIR In a mixture of ideal gases, each gas exerts a pressure as if it alone was the only gas present in the container. p1 = n1RT/V and p2 = n2RT/V Thus with two gases in a container we have PTotal = p1 + p2 where the pressure exerted by each gas is known as its Partial Pressure (p1 , p2 ) PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn