正在加载图片...
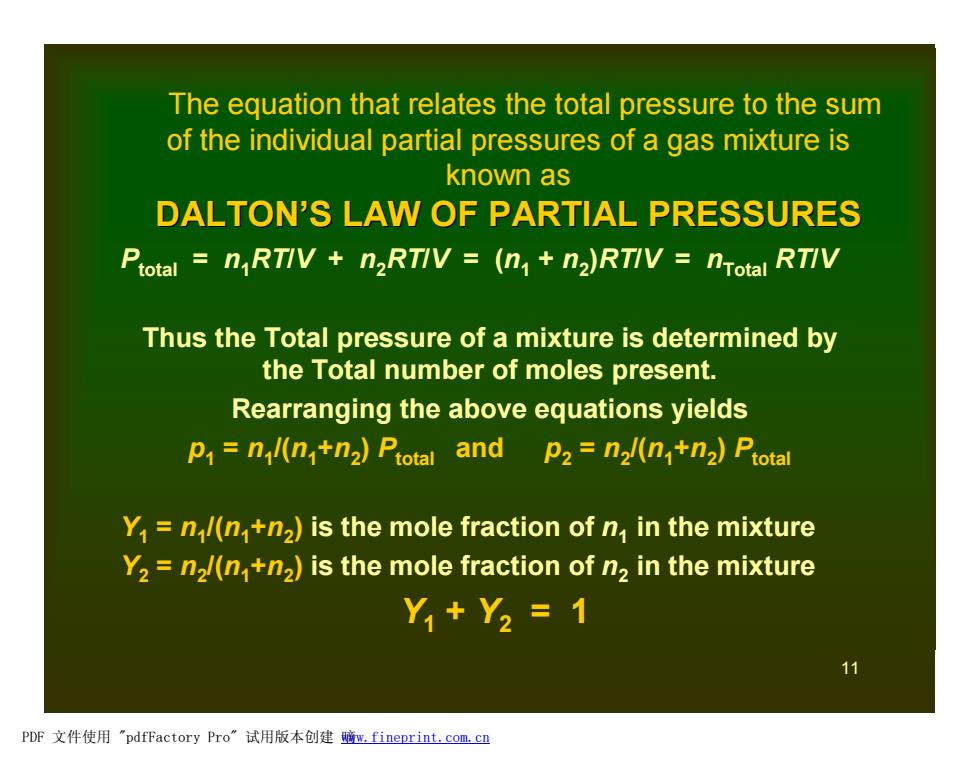
The equation that relates the total pressure to the sum of the individual partial pressures of a gas mixture is known as DALTON'S LAW OF PARTIAL PRESSURES Ptotal nRTIV n2RTIV =(n+n2)RTIV nTotal RTIV Thus the Total pressure of a mixture is determined by the Total number of moles present. Rearranging the above equations yields p=nl(n+n2)Ptotal and p2=n2l(n+n2)Ptotal Y1 n/(n+n2)is the mole fraction of n in the mixture Y2 n2/(n+n2)is the mole fraction of n2 in the mixture Y,+Y2=1 11 PDF文件使用"pdfFactory Pro”试用版本创建脑m,fineprint.com,cn 11 The equation that relates the total pressure to the sum of the individual partial pressures of a gas mixture is known as DALTON’S LAW OF PARTIAL PRESSURES Ptotal = n1RT/V + n2RT/V = (n1 + n2 )RT/V = nTotal RT/V Thus the Total pressure of a mixture is determined by the Total number of moles present. Rearranging the above equations yields p1 = n1 /(n1+n2 ) Ptotal and p2 = n2 /(n1+n2 ) Ptotal Y1 = n1 /(n1+n2 ) is the mole fraction of n1 in the mixture Y2 = n2 /(n1+n2 ) is the mole fraction of n2 in the mixture Y1 + Y2 = 1 PDF 文件使用 "pdfFactory Pro" 试用版本创建 嘀www.fineprint.com.cn