正在加载图片...
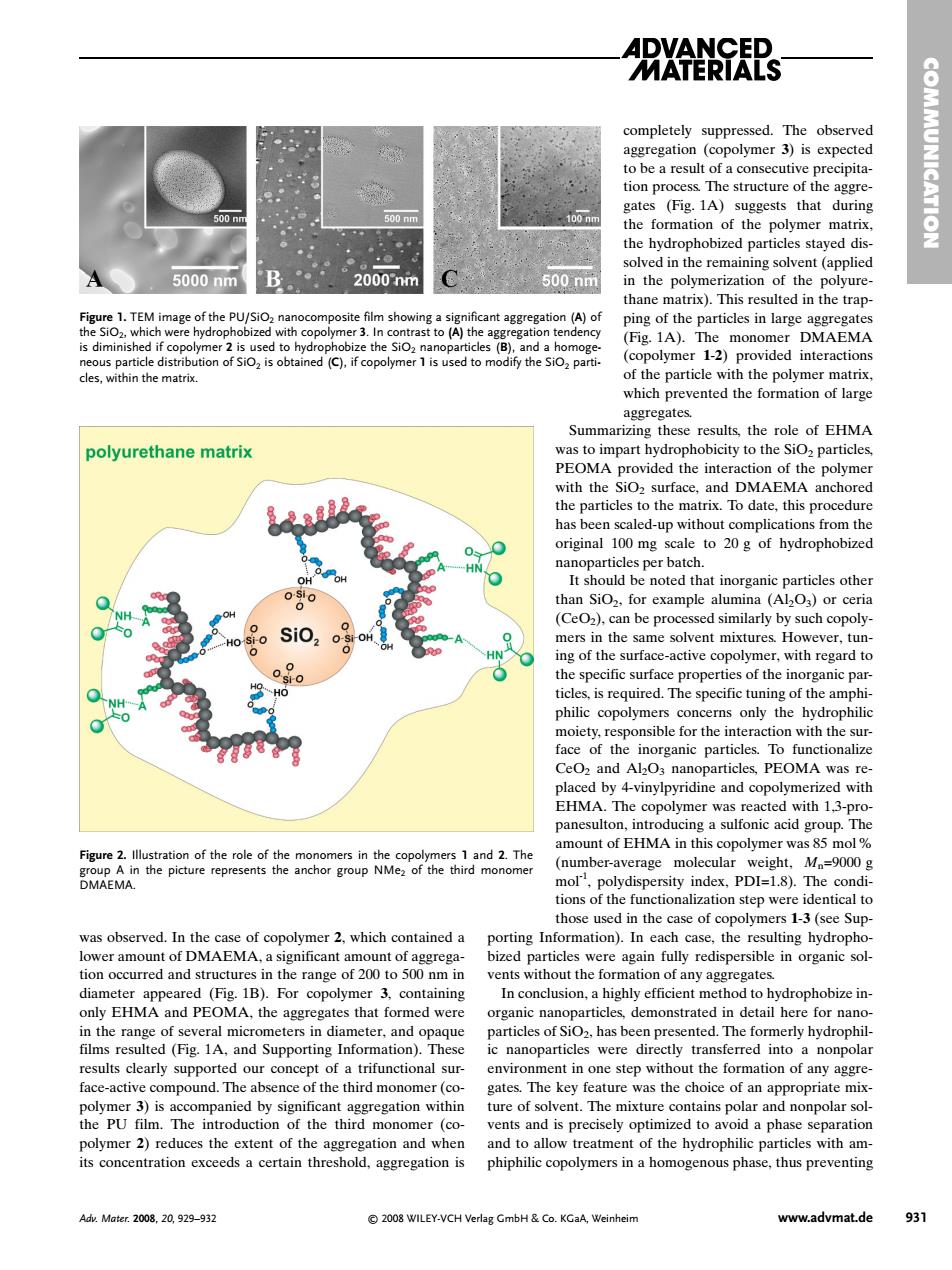
ARX思s completely suppressed.The observed tion 、The structure of gates (Fig.1A)suggests that during the t on of the poly ymer ma solved in the remaining solvent (a 5000nm 2000nm 500nm in the polymerization of the polyure- TE ast to (A)the ag (Fi.IA).The monomer DMAEMA 12 cles,within themat Summa rizing the e of EHMA polyurethane matrix y to with the SiO surface.and DMAEMA anchored particles to the ma atrix.To date.this pr e noted at inorganic (Ce)can be processed similarly by such copoly- mers in the same solvent mixtures However,tun ing of the surfa philic only the hydrophili sible for the int Ceoz and AbOs nanoparticles.PEOMA was re The 13e amount of EHMA in this copolymer was 85 mol DMAM he picture (number-average molecular M=9000g those used in the case of copolymers 1-3(see Sup porting Information).In ea case,the sulting hydrophe tommoeands in organic so gates In conclusion.a highly efficient method to hydrophobize in- organic nanoparticles,demonstrated in detail here for nane results clearly supported our concept of a trifunctional sur environment in one step without the formation of any aggre face-ac compound.Th othe third m omer (o Key was the choice of an appropr vents and is pre and to allow treatment of the hydrophilic particles with am- phiphilic copolymers in a homogenous phase,thus preventing Adv.Mater2008,20,929-932 2008 WILEY-VCH Verlag GmbH Co.KGaA,Weinhein www.advmat.de 931 was observed. In the case of copolymer 2, which contained a lower amount of DMAEMA, a significant amount of aggregation occurred and structures in the range of 200 to 500 nm in diameter appeared (Fig. 1B). For copolymer 3, containing only EHMA and PEOMA, the aggregates that formed were in the range of several micrometers in diameter, and opaque films resulted (Fig. 1A, and Supporting Information). These results clearly supported our concept of a trifunctional surface-active compound. The absence of the third monomer (copolymer 3) is accompanied by significant aggregation within the PU film. The introduction of the third monomer (copolymer 2) reduces the extent of the aggregation and when its concentration exceeds a certain threshold, aggregation is completely suppressed. The observed aggregation (copolymer 3) is expected to be a result of a consecutive precipitation process. The structure of the aggregates (Fig. 1A) suggests that during the formation of the polymer matrix, the hydrophobized particles stayed dissolved in the remaining solvent (applied in the polymerization of the polyurethane matrix). This resulted in the trapping of the particles in large aggregates (Fig. 1A). The monomer DMAEMA (copolymer 1-2) provided interactions of the particle with the polymer matrix, which prevented the formation of large aggregates. Summarizing these results, the role of EHMA was to impart hydrophobicity to the SiO2 particles, PEOMA provided the interaction of the polymer with the SiO2 surface, and DMAEMA anchored the particles to the matrix. To date, this procedure has been scaled-up without complications from the original 100 mg scale to 20 g of hydrophobized nanoparticles per batch. It should be noted that inorganic particles other than SiO2, for example alumina (Al2O3) or ceria (CeO2), can be processed similarly by such copolymers in the same solvent mixtures. However, tuning of the surface-active copolymer, with regard to the specific surface properties of the inorganic particles, is required. The specific tuning of the amphiphilic copolymers concerns only the hydrophilic moiety, responsible for the interaction with the surface of the inorganic particles. To functionalize CeO2 and Al2O3 nanoparticles, PEOMA was replaced by 4-vinylpyridine and copolymerized with EHMA. The copolymer was reacted with 1,3-propanesulton, introducing a sulfonic acid group. The amount of EHMA in this copolymer was 85 mol% (number-average molecular weight, Mn=9000 g mol-1, polydispersity index, PDI=1.8). The conditions of the functionalization step were identical to those used in the case of copolymers 1-3 (see Supporting Information). In each case, the resulting hydrophobized particles were again fully redispersible in organic solvents without the formation of any aggregates. In conclusion, a highly efficient method to hydrophobize inorganic nanoparticles, demonstrated in detail here for nanoparticles of SiO2, has been presented. The formerly hydrophilic nanoparticles were directly transferred into a nonpolar environment in one step without the formation of any aggregates. The key feature was the choice of an appropriate mixture of solvent. The mixture contains polar and nonpolar solvents and is precisely optimized to avoid a phase separation and to allow treatment of the hydrophilic particles with amphiphilic copolymers in a homogenous phase, thus preventing COMMUNICATION Adv. Mater. 2008, 20, 929–932 © 2008 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim www.advmat.de 931 Figure 1. TEM image of the PU/SiO2 nanocomposite film showing a significant aggregation (A) of the SiO2, which were hydrophobized with copolymer 3. In contrast to (A) the aggregation tendency is diminished if copolymer 2 is used to hydrophobize the SiO2 nanoparticles (B), and a homogeneous particle distribution of SiO2 is obtained (C), if copolymer 1 is used to modify the SiO2 particles, within the matrix. Figure 2. Illustration of the role of the monomers in the copolymers 1 and 2. The group A in the picture represents the anchor group NMe2 of the third monomer DMAEMA