正在加载图片...
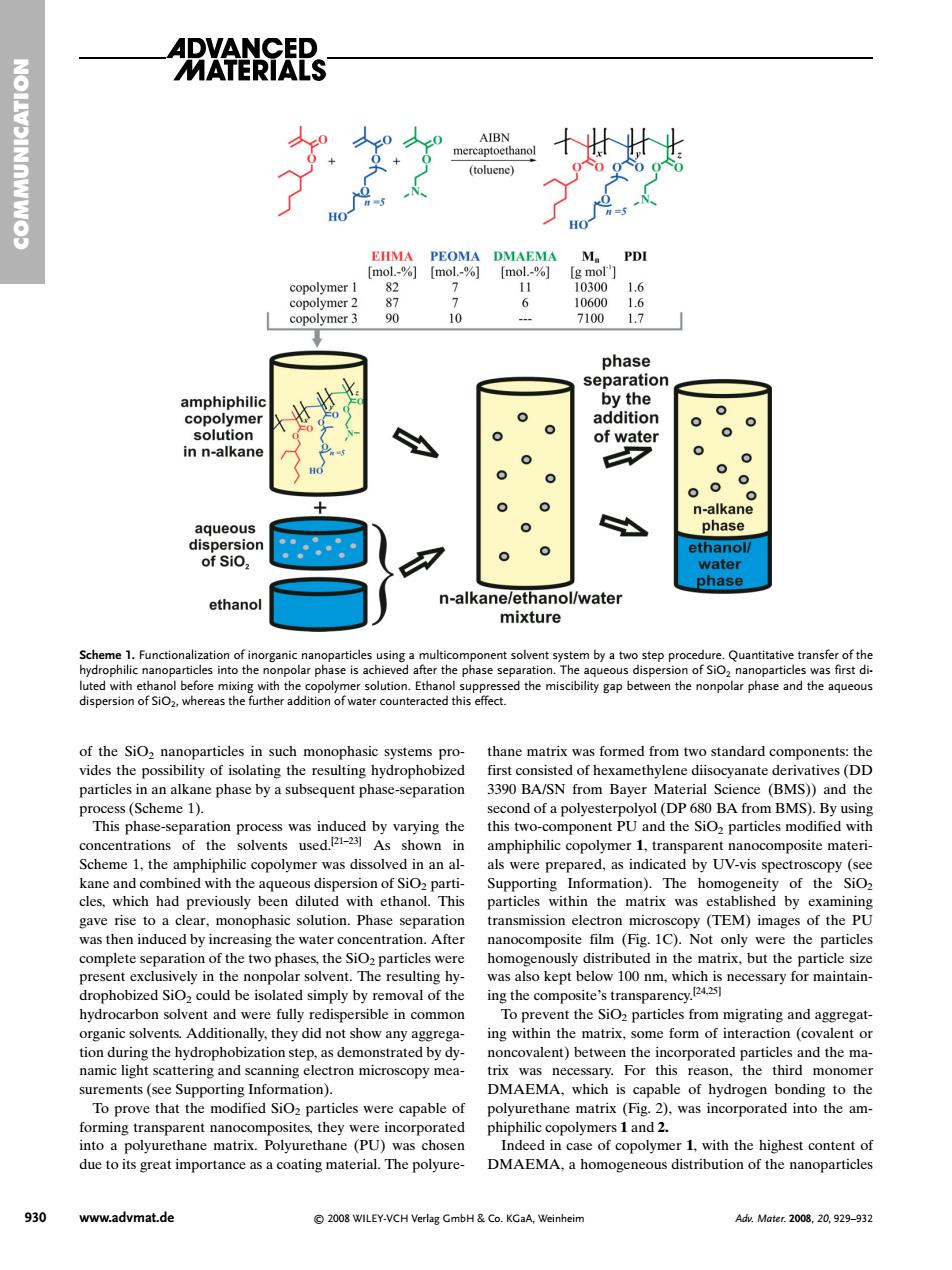
AERTAIS anma对MPo opolyme copolymer 3 9 phase y the olyme in n-alkane aqueous of Sio ethanc n-al ane /wate nixt re d the miscibility gap between the nonpolar phase and the aqueous ion pr was induced by varying the Scheme 1.the amphiphilic co rmer was dissolved in anal amphiphiue opoymer tansparent nancm and the SiO particles modific le previously particle n the mat nx was TEM) th DI was then induced by increasing the water.After (Fig.IC).Not onwere the particles complete separation of the two phases the SiO particles were ly in 10m for maintain- hvdrocarbon solvent and were fully redispersible in common To prevent the SiO particles from migrating and ag ing within the matrix.some form of interaction (covalent or tion d the hyd step.as dem sand the ma surements(see Supporting Information). DMAEMA.which is of hydrogen bondins to the To prove that the modified SiO particles were capable of tes. 2 r 1 with the highest content of due to its great importance as a coating material.The polyure- DMAEMA.a homogeneous distribution of the nanoparticles 930 www.advmat.de 2008 WILEY-VCH Verlag GmbH Co.KGaA,Weinheim Adz.Mater.2008.20.929-932of the SiO2 nanoparticles in such monophasic systems provides the possibility of isolating the resulting hydrophobized particles in an alkane phase by a subsequent phase-separation process (Scheme 1). This phase-separation process was induced by varying the concentrations of the solvents used.[21–23] As shown in Scheme 1, the amphiphilic copolymer was dissolved in an alkane and combined with the aqueous dispersion of SiO2 particles, which had previously been diluted with ethanol. This gave rise to a clear, monophasic solution. Phase separation was then induced by increasing the water concentration. After complete separation of the two phases, the SiO2 particles were present exclusively in the nonpolar solvent. The resulting hydrophobized SiO2 could be isolated simply by removal of the hydrocarbon solvent and were fully redispersible in common organic solvents. Additionally, they did not show any aggregation during the hydrophobization step, as demonstrated by dynamic light scattering and scanning electron microscopy measurements (see Supporting Information). To prove that the modified SiO2 particles were capable of forming transparent nanocomposites, they were incorporated into a polyurethane matrix. Polyurethane (PU) was chosen due to its great importance as a coating material. The polyurethane matrix was formed from two standard components: the first consisted of hexamethylene diisocyanate derivatives (DD 3390 BA/SN from Bayer Material Science (BMS)) and the second of a polyesterpolyol (DP 680 BA from BMS). By using this two-component PU and the SiO2 particles modified with amphiphilic copolymer 1, transparent nanocomposite materials were prepared, as indicated by UV-vis spectroscopy (see Supporting Information). The homogeneity of the SiO2 particles within the matrix was established by examining transmission electron microscopy (TEM) images of the PU nanocomposite film (Fig. 1C). Not only were the particles homogenously distributed in the matrix, but the particle size was also kept below 100 nm, which is necessary for maintaining the composite’s transparency.[24,25] To prevent the SiO2 particles from migrating and aggregating within the matrix, some form of interaction (covalent or noncovalent) between the incorporated particles and the matrix was necessary. For this reason, the third monomer DMAEMA, which is capable of hydrogen bonding to the polyurethane matrix (Fig. 2), was incorporated into the amphiphilic copolymers 1 and 2. Indeed in case of copolymer 1, with the highest content of DMAEMA, a homogeneous distribution of the nanoparticles COMMUNICATION 930 www.advmat.de © 2008 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim Adv. Mater. 2008, 20, 929–932 Scheme 1. Functionalization of inorganic nanoparticles using a multicomponent solvent system by a two step procedure. Quantitative transfer of the hydrophilic nanoparticles into the nonpolar phase is achieved after the phase separation. The aqueous dispersion of SiO2 nanoparticles was first diluted with ethanol before mixing with the copolymer solution. Ethanol suppressed the miscibility gap between the nonpolar phase and the aqueous dispersion of SiO2, whereas the further addition of water counteracted this effect