正在加载图片...
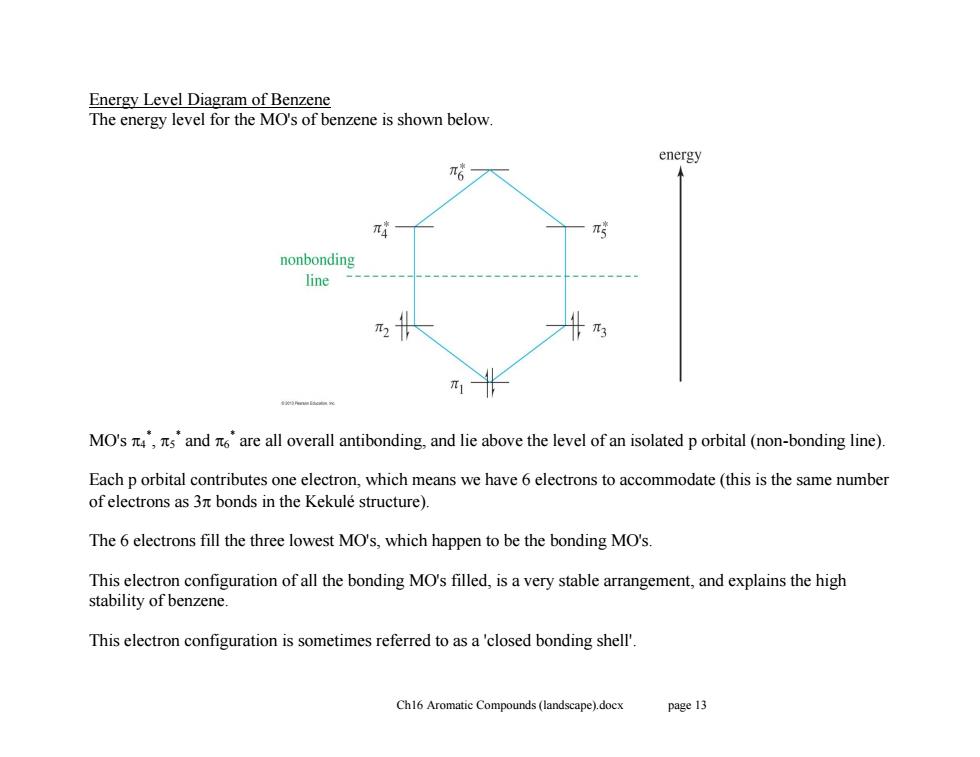
Energy Level Diagram of Benzene The energy level for the MO's of benzene is shown below energy π6 nonbonding line π3 π1 MO's,sand are all overall antibonding,and lie above the level of an isolated p orbital (non-bonding line). Each p orbital contributes one electron,which means we have 6 electrons to accommodate(this is the same number of electrons as 3nt bonds in the Kekule structure). The 6 electrons fill the three lowest MO's,which happen to be the bonding MO's. This electron configuration of all the bonding MO's filled,is a very stable arrangement,and explains the high stability of benzene This electron configuration is sometimes referred to as a'closed bonding shell'. Ch16 Aromatic Compounds(landscape).docx page 13 Ch16 Aromatic Compounds (landscape).docx page 13 Energy Level Diagram of Benzene The energy level for the MO's of benzene is shown below. MO's 4 * , 5 * and 6 * are all overall antibonding, and lie above the level of an isolated p orbital (non-bonding line). Each p orbital contributes one electron, which means we have 6 electrons to accommodate (this is the same number of electrons as 3 bonds in the Kekulé structure). The 6 electrons fill the three lowest MO's, which happen to be the bonding MO's. This electron configuration of all the bonding MO's filled, is a very stable arrangement, and explains the high stability of benzene. This electron configuration is sometimes referred to as a 'closed bonding shell